The reactivation of hepatitis B virus (HBV) infection is defined as an increase in HBV replication in a patient with an inactive or resolved hepatitis (usually accompanied by increased serum transaminase levels). Most reported cases have occurred in patients with hematologic malignancies after chemotherapy.1–3 Recently, there have been reports of HBV reactivation in patients with rheumatoid arthritis after treatment with biological therapies such as rituximab (RTX)4,5 (Table 1). Although most cases of HBV reactivation have occurred in patients with serology indicating chronic HBV infection, patients with resolved HBV infection can develop this complication.5
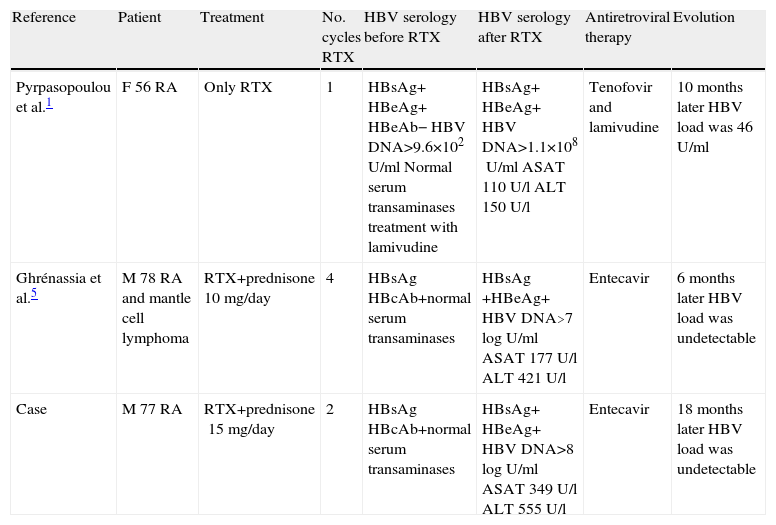
Previous Cases of HBV Reactivation in RA Patients Following Treatment With RTX.
| Reference | Patient | Treatment | No. cycles RTX | HBV serology before RTX | HBV serology after RTX | Antiretroviral therapy | Evolution |
| Pyrpasopoulou et al.1 | F 56 RA | Only RTX | 1 | HBsAg+ HBeAg+ HBeAb− HBV DNA>9.6×102U/ml Normal serum transaminases treatment with lamivudine | HBsAg+ HBeAg+ HBV DNA>1.1×108U/ml ASAT 110U/l ALT 150U/l | Tenofovir and lamivudine | 10 months later HBV load was 46U/ml |
| Ghrénassia et al.5 | M 78 RA and mantle cell lymphoma | RTX+prednisone 10mg/day | 4 | HBsAg HBcAb+normal serum transaminases | HBsAg +HBeAg+ HBV DNA>7logU/ml ASAT 177U/l ALT 421U/l | Entecavir | 6 months later HBV load was undetectable |
| Case | M 77 RA | RTX+prednisone15mg/day | 2 | HBsAg HBcAb+normal serum transaminases | HBsAg+ HBeAg+ HBV DNA>8logU/ml ASAT 349U/l ALT 555U/l | Entecavir | 18 months later HBV load was undetectable |
F: female, M: male.
We report the case of a 77 year old patient with a history of seropositive and erosive rheumatoid arthritis of 12 years of evolution, receiving the first cycle of RTX in February 2008. Serology for HBV in 2001 was HBsAg negative and anti-HBc positive, compatible with resolved hepatitis B. The patient received methotrexate from 2002 to 2004 and the treatment was stopped by the appearance of oral ulcers. In 2005, treatment was started with infliximab 3mg/kg every 8 weeks with good response; after one year of treatment, the patient presented a lack of response in March 2006 and it was suspended due to multiple episodes of lung infections and switched to etanercept 50mg weekly. From 2005 to 2008, serum levels of ALT transaminase (GPT) ansd AST (GOT), remained stable, but new virus serology was not requested. Due to poor control of symptoms (disease activity score [DAS] 28 joints 6.43) the patient was treated with RTX (2×1000) in February 2008. He received the second cycle of RTX in August 2008. After treatment, the DAS 28 was 3.79, but in July 2009 the patient had anicteric hepatitis with ALT (GPT) 555U/L, AST (GOT) 349U/l, GGT 81U/l, total bilirubin 0.7mg/dl and HBsAg+, HBeAg+, albumin35mg/dl, 70% TP, 99000platelets/mm3 and HBV DNA was >8logU/ml (virus genotype was A). This data indicated the presence of a reactivation of chronic hepatitis B with a moderate degree of hepatocellular failure. At this point the patient quickly was sent to the hepatologist and began treatment with entecavir. After one month of therapy, HBV was DNA down to 5.4logU/ml. Five months later HBV-DNA was only 1.74U/ml (3.24logU/ml), but remained positive until February 2011. The biochemical response was faster, with normalization of transaminases after only 8 months of therapy.
HBV reactivation after immunosuppressive treatment is a serious adverse effect which can be identified and prevented. There have been various guidelines and recommendations but the management of HBV occult infection with only anti-HBc positivity remains unclear today.6,7 The consensus on RTX use in rheumatoid arthritis recommends monitoring of transaminases and/or HBV DNA8 in patients without active HBV with HBsAg negative but anti-HBc-positive antibodies, as in our patient. In patients who had increased levels of HBV DNA during therapy with RTX, one should consider associating antiretroviral therapy. The exact mechanism of HBV reactivation is unknown and although it is not a very common complication, there have been cases of fulminant hepatitis that were seen even after 6 months of cessation of therapy with RTX.9 Prophylactic treatment with antiretroviral therapy significantly reduces the incidence of HBV reactivation after treatment with RTX.10 In conclusion, strict monitoring should follow liver biochemistry, HBsAg, HBV-DNA in the anti-HBc positive and HBsAg negative patients during and 6 months after treatment with RTX.
Please cite this article as: Salman-Monte TC, Lisbona MP, García-Retortillo M, Maymó J. Reactivación del virus de la hepatitis B en un paciente con artritis reumatoide tras el tratamiento con rituximab. Reumatol Clin. 2014;10:196–197.