To design a strategy for the early detection and referral of patients with possible spondyloarthritis based on recommendations developed, agreed upon, and directed to primary care physicians.
MethodsWe used a modified RAND/UCLA methodology plus a systematic literature review. The information was presented to a discussion group formed by rheumatologists and primary care physicians. The group studied the process map and proposed recommendations and algorithms that were subsequently submitted in two Delphi rounds to a larger group of rheumatologists and primary care physicians. The final set of recommendations was derived from the analysis of the second Delphi round.
ResultsWe present the recommendations, along with their mean level of agreement, on the early referral of patients with possible spondyloarthritis. The panel recommends that the study of chronic low back pain in patients under 45 years could be performed in four phases (1) clinical: key questions, (2) clinical: extra questions, (3) physical examination, and (4) additional tests.
ConclusionsThe level of agreement with these simple recommendations is high. It is necessary to design strategies for the education and sensitization from rheumatology services to maintain an optimal collaboration with primary care and to facilitate referral to rheumatology departments.
Diseñar una estrategia de detección y derivación precoz de pacientes con posible espondiloartritis mediante el desarrollo de recomendaciones consensuadas dirigidas a los médicos de Atención Primaria (AP).
MétodosSe utilizó una metodología modificada de RAND/UCLA y revisión sistemática de la literatura. Se seleccionó un grupo de discusión formado por reumatólogos y médicos de AP. Se estudió el mapa del proceso y se propusieron recomendaciones y algoritmos que fueron sometidos a 2 rondas Delphi para evaluar el grado de aceptación y preferencia de criterios en un grupo amplio de reumatólogos y médicos de AP. Del análisis de la segunda ronda Delphi se extrajeron las recomendaciones finales.
ResultadosSe presentan recomendaciones, junto con su grado medio de acuerdo, para la derivación rápida de pacientes con sospecha de espondiloartritis. En concreto, se recomienda investigar el dolor lumbar crónico en menores de 45 años en 4 fases: 1) clínica: preguntas clave; 2) clínica: preguntas extra; 3) exploración física, y 4) pruebas complementarias. Se debe derivar a Reumatología si existen: 1) dolor lumbar inflamatorio; 2) signos indicativos de espondiloartritis, o 3) HLA B27 positivo, elevación de proteína C reactiva o signos radiológicos de sacroilitis. Se incluyen recomendaciones sobre el proceso de derivación y otras adicionales.
ConclusionesEl grado de acuerdo con estas sencillas recomendaciones es amplio. Es necesario diseñar estrategias de formación y sensibilización desde los servicios de Reumatología para mantener una óptima colaboración de AP en la identificación de los casos y facilitar que los servicios de Reumatología estén preparados para asumir las derivaciones.
Axial Spondyloarthritis (SpA) is a chronic inflammatory disease that primarily affects the spine and sacroiliac joints, which basically evolves from an unaffected form (non radiological axial SpA) to another with radiographic sacroiliitis (ankylosing spondylitis [SA]) and can be known using on conventional X-rays.1,2
It has been estimated that its prevalence is about 0.7%.3 It is a known fact that the disease commonly debuts insidiously, usually before 40 years of age, with chronic inflammatory low back pain. Axial SpA and AS can be diagnosed using a series of simple clinical and radiological criteria, based on the HLA B27. However, it can take between 7 and 9 years after the onset of symptoms until a diagnosis is established.4,5
This delay in diagnosis leads to a delay in establishing the most appropriate treatment for each patient and the adverse consequences of untreated disease may diminish the quality of life of the patient, causing prolonged sick leave and increasing the economic burden of the process, in addition to promoting structural damage associated with the presence of untreated disease in the first years of evolution.6,7 That is why strategies are being designed for referral of early stage patients with axial symptoms to Rheumatology clinics, which would help shorten diagnosis time and optimize the therapeutic management of these patients in the earliest stages of4,8–12 disease.
The RADAR study12 found that inflammatory LBP is the most commonly used criterion and, simultaneously, the one that yielded the most results, in order to establish a mechanism for referral from primary care (PC) to Rheumatology. There is, however, no consensus recommendation with PC to specify what criteria must be evaluated in a patient with chronic back pain in order to decide an appropriate referral to Rheumatology.
The ultimate objective of this document is to improve the quality of care for patients with chronic, inflammatory low back pain and SpA by creating validated and easy to use resource that does not interrupt the consultation and that ultimately benefits the patient. This resource should include criteria for suspicion, research algorithms and recommendations for referral to Rheumatology. The target group who should use this resource are primary care physicians (PCP).
MethodsA modified RAND methodology13 was used, with group discussions and14 Delphi technique to evaluate the degree of acceptance of the recommendations and the preference criteria.
A panel of 4 SpA expert rheumatologists and 4 PCP, all Spaniards, moderated by a methodologist, held a meeting to determine the scope, users and the set of existing criteria, and to identify the difficulties of assessment and referral of patients with suspected SpA. The group followed the following script: (1) what are the available criteria for suspecting/referring inflammatory back pain? (2) how are the available criteria evaluated? (3) what is the current referral process and the systematic evaluation of low back pain in primary care?, weighing the difficulty of assessing the criteria available in this context, (4) what parameters allow an informed selection of the criteria to recommend?, and (5) solutions or necessities to improve the implementation of criteria (training, research, etc.).
In order to document the suitability of recommendations and facilitate the decisions of the panelists, a systematic review of the literature on low back pain in primary care was undertaken and the results were condensed into evidence tables. This review identified existing criteria and those proposed by the panel, as well as their performance. In particular, the objective was to understand their sensitivity and specificity, as they would become screening instruments. The search strategy for the review is available in an Annex 1; it basically included synonyms for “Spondyloarthritis AND Low back pain AND (sensitivity and specificity) AND primary care.” The panelists were queried on the ease of collecting the data in PC, in particular if the data is included in the usual history and whether the criterion can be interpreted correctly, that is, if there is a good agreement between what Rheumatologists and PCP think.
All of the information collected was summarized in a report and a survey format was prepared for the vote by 25 professionals, including rheumatologists and PCP. The first Delphi round was used to prioritize items and gather comments and views on the proposals, and the second to define the degree of agreement. The conflicting items (under agreement or excessive variability) were discussed and reformulated for the second round. Each recommendation comes as a result with an average degree of agreement in the second round. The full study was conducted throughout 2013.
ResultsA literature search was conducted and a second, manual search of the publications obtained led researchers to 12 studies which were retrieved for detailed review. Evidence tables were built by extracting performance parameters of different clinical features in the diagnosis of SpA (Table 1). Table 2 summarizes the reproducibility and diagnostic performance parameters described by Rudwaleit et al. in 2004 from15 different studies. The Annex 1 also includes a table in which the data available for each criterion proposed by the panel is summarized.
Summary of the Evidence of the Items Selected in the Systematic Review for Referral Criteria.
| Study | Population | Criteria for referral (referral) | Diagnosis of SpA(No. and %) | Diagnostic utility | Comments |
|---|---|---|---|---|---|
| Brandt et al., 200723 | (No.=350)Low back pain (>3 months) in <45 years | At least one of the following:inflammatory lumbar paina,HLA-B27 +,sacroiliitis (image) | Global: 159/350=45%Criteria: 55/161=35%>Criteria: 102/163=64%+HLA sacroiliitis: 28/33=85%HLA+IBP: 33/57=%IBP+sacroiliitis: 10–26=% | Criteria:S=35%; E=45%; LR=0.62+>Criteria:S=64%; E=68%; LR=2.01+ | The use of IBP for screening requires experienceHLA-B27: always in combination with other parameters |
| Poddubnyy et al., 20119 | (No.=560)Low back pain (>3 months) in <45 years | (No.=318)Strategy 1: ≥1:inflammatory LBPHLA-B27+Sacroiliitis (image) | Strategy 1: 133/318=42%AS: 82/318 =26%: ASNo radiological SPA: 51/318=16% | Strategy 1bIBP:S=77%; E=23%; =1+Sacroiliitis:S=%; E=52%; LR=1.4HLA-B27:S=62%; E=68%; LR=1.9 | Increased likelihood of diagnosis>no. criteriaPoor agreement PCP/IBP rheumatologists and radiological sacroiliitisStrategy 1 is an effective and reliable method for screening SPA in patients with axial low back pain in PC |
| (No.=242)Strategy 2: ≥2:Inflammatory back painHLA-B27 +Sacroiliitis (image)Family history of ASGood response to NSAIDs | Strategy 2: 89/242=37%AS: 55/242=23%:No radiological SPA: 34/242=14%No significant difference | Strategy 2bIBP:S=83%; E=10%; + LR=0.9NSAIDs:S=61%; E=33%; + LR=0.9HLA-B27:S=70%; E=58%; LR=1.6Sacroiliitis: S=55%; E=72%; LR +=2HTA family: S=24%; E=84%; LR=1.4 | |||
| Rudwaleit et al., 200624 | (No.=213: 101 AS and 112 low back pain)Low back pain (≥3 months) in ≤50 yearsObjective: value features and combinations IBP | Combination 1:≥2:Morning stiffness >30mImprovement with exercise but not with restPain, wakes up in the 2nd half of the nightAlternate buttock painCombination 2:≥3 of the above | Diagnostic value for IBPCombination 1: S=70%; E=81%; + LR=3.7 | Limitations:Convenience sampleCross DesignExaminer not blind to diagnosisNeeds validation in prospective study | |
| Blend 2+LR=12.4 | |||||
| Hermann et al., 200925 | (No.=92)Referral from PC<45 years who met Calin criteria for IBPIBP Calin: pain ≥4m ≥3<40 yearsInsidious onsetImprovement with exerciseMorning stiffness | Blend 1Pain at nightSI pain palpationIBP as Calin criteriaCervical neck pain on movement | Diagnostic RheumatologistSPA: 33%Noninflammatory DL: 67% | Isolated LR parameters:Night pain: 3.3Improved exercise no rest: 2.1Pain exploration SI: 3.8HLA-B27 4.1Blend 1. Parameters associated with both SPA and mechanical DLS=90%; E=95%; LR=16.8 | ROC curves stepwise regression was performed to study association between clinical parameters and diagnosis of SPA, and to calculate the S and E of the 2 combinationsIsolated clinical parameters are of little use. Only when data from involvement of the cervical spine are added S and E values above 90% are achievedThe combination includes parameters not used in PC (scanning mobility of spine and sacroiliac) |
| Combination 2:Pain at nightSI pain palpationIBP as Calin criteria | Combination 2. Parameters associated only with SPAS=60%; E=88%; +LR=4.8 | ||||
| Braun et al., 20118 | (No.=322)Referral from PCLow back pain <45 years and lasting >2m | Inclusion criteria:Morning stiffness >30minImprovement with exercise, not restPain at nightResponse to NSAIDsCombination of these parameters in different cohortsAdditional criteria:Alternate buttock painFamily history SPAExtraspinal manifestations: arthritis, enthesitis, psoriasis, IBD and HLA-B27 | Diagnostic rheumatologist:SPA: 113 (35%)AS: 47SPA no Rx: 66Back pain, non SPA: 209 (65%) | Isolated Criteria: LR + S SPARigidity >30min: 35%; 1.1Improved exercise no rest: 78%; 1.3Pain at night: 58%; 1.7Answer NSAIDs: 94%; 1.8Alternating buttock pain: 25%; 2.2Ant enthesitis: 15%; 1.9Ant arthritis: 11%; 2.5Age <35: 77%; 1.4HLA-B27: 35%; 3.9≥3 of the above: 85%; 1.7Greater power of discrimination in regression analysis:Age ≤35 yearsPain at nightAlternate buttock painResponse to NSAIDsImproved exercise not restDiagnostic value (S, E) of the combinations of the latter≥4:48% and 86%≥3:78% and 46%≥2:96% and 17% | ProspectiveIsolates criteria have little value in PCThe combinations have greater power of discriminationRemission cutoff age: less than in other studies |
| Keeling et al., 201226 | Development and validation of a questionnaire on IBP in PC(No.=286) | DomainsMorning stiffnessPain at nightDiurnal variation of symptomsPeripheral joint involvementResponse to exerciseReply to rest | IBP=220DLM=66IBP Association (regression):Diurnal variationResponse ExerciseReply to rest | + S and LR for individual items:Stiffness: 48%, 1.9Pain at night: 51%; 1.3Diurnal variation: 49%, 5.9Response exercise: 53%, 1.8Reply to rest: 60%, 1.4 | Best utility for ‘diurnal variation’ (S 49% and LR+5.9)The results do not improve with the combination of itemsMorning stiffness does not discriminate well between IBP and nonspecific DLThe “diurnal variation” is an important feature of IBP and indicates remission, especially when it comes to young patients with HLA+ |
| Sieper et al., 201212 | Entry criteria: chronic LBP (>3m) in <45 | Strategy 1. Either:IBPHLA-B27Sacroiliitis (image)Strategy 2: ≥2IBPHLA-B27Sacroiliitis (image)Ant family axial SpAResponse to NSAIDsExtra-articular manifestations | No.=1072 patients referredStrategy 1: 504Strategy 2: 568Seen by a rheumatologist No.=1.049Diagnosis of SpA (rheumatologist)Strategy 1: 176 (36%)Strategy 2: 221 (40%) | Individual Criteria: LR+SSacroiliitis: 76%, 19.9HLA-B27: 66%, 3.3M. extraarticular: 55%, 2.2IBP: 94%; 1.3Ant fam of SpA: 13%, 1.5Response to NSAIDs: 69%, 1.4Combinations>performanceSacroiliitis IBP+: 72%, 30.8Two of:Sacroiliitis, HLA IBP: 86%, 5.4Extraarticular, IBP, NSAIDs: 85%, 1.6 | International multicenter study.Strategy 1: Similar to 2 results and simplerIBP is the criterion most used branch and PCP-rheumatologist good agreementThe combination with>diagnostic performance is the use of 2 of the following 3 criteria: IBP, response to NSAIDs and extra-articular manifestationsThis combination has a 85% S and LR+1.6, with good agreement for IBP and extra-articular (85% and 77%) events, but relatively low for the response to NSAIDs |
NSAIDs: NSAIDs; PC: primary care; Ant: antecedent; BP: back pain; IBP: inflammatory back pain; MBP: mechanical back pain; E: specificity; AS: ankylosing spondylitis; IBD: inflammatory bowel disease; SpA: spondylitis; HLA: human leukocyte antigen; HT: hypertension; ROC: receiver operating curve; LR: likelihood ratio; S: sensitivity; SPA: spondyloarthritis.
Sensitivity, Specificity and Positive Likelihood Ratios of Clinical and Laboratory Characteristics of Patients With Ankylosing Spondylitis, Back Pain Controls, Patients With Any Type of Spondyloarthritis or Any Control.
| Patient groups and group size | ||||||
|---|---|---|---|---|---|---|
| S (%) | E (%) | LR+ | AS(n) | Control lumbar pain (n) | All SpA (n) | Control (n)a |
| Inflammatory lumbar pain | ||||||
| 95 | 76 | 4.0 | 42 | 21 | – | |
| 38 | 100 | 3.1 | 21 | – | 83 | |
| 65 | 79 | 3.6 | 27 | 422 | – | |
| 71 | 75 | 3.1 | – | – | 774 | |
| 75 | 80 | 101 | 112 | – | ||
| 76 | ||||||
| Alternating buttock pain | ||||||
| 20 | 97 | 6.6 | 403 | 674 | ||
| 39 | 98 | 19.5 | 124 | 1964 | ||
| 20 | 89 | 1.8 | 44 | 29 | 104 | 75 |
| 43 | 95 | 9.6 | 218 | 1242 | ||
| 43 | 100 | – | 101 | 112 | ||
| 32 | 97 | 10.4 | 105 | 163 | ||
| 37 | 98 | 3.7 | ||||
| 40 | 90 | 4.0 | ||||
| Talalgia (enthesitis) | ||||||
| 16 | 90 | 1.6 | 70 | 32 | ||
| 37 | 89 | 3.4 | 403 | 674 | ||
| 25 | 90 | 2.5 | 44 | 29 | 104 | 75 |
| 52 | 92 | 6.5 | 124 | 1964 | ||
| 47 | 94 | 7.8 | 218 | 1242 | ||
| 50 | 96 | 12.5 | ||||
| 52 | 93 | 7.4 | 105 | 163 | ||
| 37 | 89 | 3.4 | ||||
| Peripheral arthritis | ||||||
| 41 | 94 | 6.8 | 70 | 32 | ||
| 40 | 90 | 4.0 | 403 | 674 | ||
| 44 | 95 | 8.8 | 44 | 29 | 124 | 1964 |
| 42 | 91 | 4.7 | 218 | 1242 | ||
| 62 | 100 | – | ||||
| 26 | 98 | 13 | 105 | 163 | ||
| – | – | – | ||||
| 40 | 90 | 4.0 | ||||
| Dactylitis | ||||||
| 18 | 96 | 4.5 | 403 | 674 | ||
| 27 | 99 | 27 | 124 | 1964 | ||
| 24 | 96 | 6 | 218 | 1242 | ||
| 12 | 98 | 6 | 105 | 163 | ||
| 18 | 96 | 4.5 | ||||
| Anterior uveitis | ||||||
| 10 | 100 | – | 70 | 32 | ||
| 19 | – | – | 42 | 12 | ||
| 22 | 97 | 7.3 | 403 | 674 | ||
| 14 | 99 | 1 | 676 | 124 | 1964 | |
| 13 | 99 | 13 | 218 | 1242 | ||
| 4 | 100 | – | 105 | 163 | ||
| 21 | – | – | ||||
| 22 | 97 | 7.3 | ||||
| Psoriasis | ||||||
| 17 | – | – | 807 | |||
| 1.2 | – | – | 676 | |||
| 10 | 96‡ | 2.5 | ||||
| IBD | ||||||
| July | – | – | 828 | |||
| 1.7 | – | – | 676 | |||
| 4 | 99 | 4. | ||||
| A family history of AS, reactive arthritis, IBD, psoriasis, uveitis | ||||||
| July | 100 | – | 70 | 32 | 104 | 75 |
| 31 | 93 | 4.4 | 403 | 674 | ||
| 32 | 95 | 6.4 | 44 | 29 | 218 | 1242 |
| 36 | 97 | 12 | ||||
| 20 | 100 | – | 676 | 105 | 163 | |
| 15 | 99 | 1 | ||||
| 10 | – | – | ||||
| 32 | 95 | 6.4 | ||||
| Response to NSAIDs | ||||||
| 77 | 85 | 5.1 | 69 | 769 | ||
| 71 | 75 | 2.8 | 218 | 1242 | ||
| 61 | 80 | 3.1 | 676 | 105 | 163 | |
| 64 | – | – | ||||
| 77 | 85 | 5.1 | ||||
| Elevated acute phase reactants (CRP) | ||||||
| 49 | 100 | – | 70 | 32 | ||
| 69 | 67 | 3 | 42 | 12 | ||
| 39 | – | – | 443 | |||
| 38 | – | – | 149 | 112 | ||
| 75 | – | – | 70 | |||
| 51 | 75 | 2. | 101 | |||
| 56b | – | – | ||||
| 50 | 80 | 2.5 | ||||
| HLA-B27 | ||||||
| 96 | 96 | 24 | 40 | 906 | ||
| 88 | 92 | 11 | 75 | 75 | ||
| 83 | 95 | 16.6 | 70 | 32 | ||
| 88 | – | 42 | 12 | 1871 | ||
| – | 91 | |||||
| 89 | 94 | 14.8 | 101 | 112 | ||
| 90c | 90 | 9.0 | ||||
| MR | ||||||
| 93 | 100 | – | 25 | 12 | 1 | |
| 54 | 83 | 3.1 | 12 | 24 | ||
| 83 | 93 | 11.8 | 36 | 53 | 36 | |
| 94 | 100 | – | 20 | 17 | ||
| 90 | – | – | 41 | |||
| 90 | 90 | 9.0 | ||||
Each line corresponds to the result of a study. The final line in bold corresponds to the pooled value.
NSAIDs: NSAIDs; E: specificity; AS: ankylosing spondylitis; IBD: inflammatory bowel disease; SpA: spondylitis; CRP: C-reactive protein; LR: likelihood ratio; MR: Magnetic resonance; Rx: radiology; S: sensitivity; ESR: erythrocyte sedimentation rate.
The sensitivity of HLA-B27 refers to SpA with axial involvement, i.e. with inflammatory back pain.
Reproduced from: Rudwaleit et al., 15 with permission from BMJ Publishing Group Ltd.
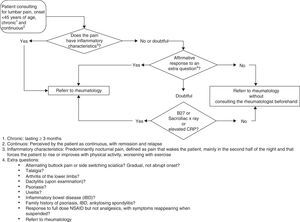
The recommendations are presented and discussed below in tabular format (Table 3), each accompanied with the degree of agreement (0–10) and an algorithm (Fig. 1).
Recommendations for Evaluation and Referral of Patients With IBP.
| Recommendation | Agreement(mean of 10) |
|---|---|
| It is recommended that, as an entry criterion, the presence of IBP in patients is investigated:• <45 years (1)• Presents chronic back pain that lasts ≥3 months (2) -• The main cause of consultation (3)• Perceived by the patient as continuous, while recognizing exacerbation and improvement (4) | (1) 8.6(2) 8.3(3) 7.6(4) 7.4 |
| It is recommended that evaluation is done in 4 phases:1. Key Questions2. Extra Questions3. Physical Examination4. Testing | 8.4 |
| In positive cases with need of any further question or examination, it is appropriate to derive a rheumatologist | |
| 1. Key Questions:• predomiant nocturnal pain, defined as pain that wakes the patient up, in the second part of the night with patient forced to rise (1)• Pain that improves with exercise and worsens with rest (2) | (1) 8.8(2) 8.0 |
| Extra questions: if the answer to the above questions is negative but leaves enough doubt whether it is IBP, should investigate:• alternating buttock pain and alternating sciatica (1)• gradual, not sudden start (2)• Response to NSAID at full dose, but not analgesics, which reappears as (3) if suspendedor assess the presence of other manifestations:• MS:Talalgia (4)IBD Arthritis (5)• non MS:Psoriasis (6), Uveitis (7) IBD (8)or inquire about a family history of:• Psoriasis (9)• IBD (10)• AS (11) | (1) 7.7(2) 7.0(3) 8.0(4) 7.0(5) 8.0(6) 7.9(7) 8.6(8) 8.1(9) 6.8(10) 6.6(11) 7.4 |
| 3. Determine the presence of dactylitis | 8.3 |
| 4. TestinfThe only evidence that could guide and should be performed in primary care, depending on availability, are:• posteroanterior sacroiliac Rx (1)• laboratory:ESR (2)CRP (3)HLAB27 (4)In areas where access to evidence or the outcome is limited, it is suggested that doubtful cases should be referred to a rheumatologist in the absence of tests (5) | (1) 8.4(2) 7.5(3) 7.9(4) 8.3(5) 7.9 |
NSAIDs: NSAIDs; IBP: inflammatory back pain; AS: ankylosing spondylitis; IBD: inflammatory bowel disease; MS: skeletal muscle; CRP: C-reactive protein; SpA: spondyloarthritis.
Algorithm for referral of patients with inflammatory back pain. According to the algorithm, the referral of patients under 45 years of age with chronic back pain should be done in 4 phases defined by key questions, extra questions, physical examination and laboratory tests. The components of each of these phases are described in the text.
The panel determined that target patients to investigate the possibility of inflammatory and SpA back pain in, and therefore those to whom these guidelines apply are those in which pain begins before age 45; lumbar pain should be the chief complaint, in which back pain is chronic, defined as lasting 3 months or more and perceived by the patient as continuous, while recognizing exacerbation and improvement periods.
What will Be the Outcome of the Patients With Inflammatory Low Back Pain?The outcome in patients with inflammatory back pain is decided according to the algorithm presented in Fig. 1. It is recommended that the evaluation of chronic low back pain in patients under 45 years could be carried out in 4 phases: (1) history: key questions, (2) clinical: extra questions, (3) physical examination, and (4) additional tests.
Key questions. Determine if the pain has any of the following characteristics, making it inflammatory back pain, which would be considered a cause for referral to rheumatology: (1) predominant night pain, defined as pain that wakes the patient mainly or preferentially during the second part of the night and requires them to wake up, and (2) improvement with exercise and worsening with rest.
Extra questions and physical examination. If the pain does not have the above characteristics but there is sufficient doubt about whether it is inflammatory pain, the clinician should investigate the following aspects: (1) on inflammatory low back pain: (a) an alternating buttock pain and sciatica that switches sides?, (b) has a gradual onset, acute and sharp and (c) pain responds to NSAIDs at doses full, but not to analgesics, and reappears (2) does the patient also have other musculoskeletal manifestations of inflammatory back pain?. Specifically investigate: (a) heel pain (in back of the heel or in their region of support), and (b) arthritis in lower extremities, (3) Does the patient present any extra-articular manifestations: (a) psoriasis, (b) uveitis, and (c) inflammatory bowel disease, and (4) does the patient's family have a history of: (a) psoriasis, (b) inflammatory bowel, or (c) AS?
This is a purely clinical phase and is not considered conclusive, even complemented with the physical examination, except for the presence of dactilitis (sausage digits).
The positive response to any of these questions or additional exploration data indicates the appropriateness of referral to a rheumatologist.
Additional testing. Additional tests may be useful in cases in which pain presents dubious inflammatory characteristics: (1) postero-anterior sacroiliac X-rays, and (2) laboratory tests: (a) erythrocyte sedimentation rate, (b) CRP, and (c) HLA-B27, depending on their availability in PC, so the algorithm may end directly at the previous step, depending on the existing protocols in the area. In areas where access to these is limited, it is suggested that doubtful cases should be referred to a rheumatologist in the absence of testing. Evaluating sacroiliac X-rays will usually be performed in the area without undergoing standardization, but providing images for assessment by a rheumatologist.
Recommendations Related to the Referral ProcessAny resource or desired process implemented in PC faces the general problem of a lack of time by the PCP. In this regard, the panel recommends that this document be translated into simple materials, despite recognizing that it is difficult to obtain documents for each of the diseases to identify and above all find a place in the PC educational curriculum, so that they become part of the standard protocols (degree of agreement 8.9/10).
Given the obstacles, and the low incidence of SpA,16 keeping PCP sensitized to the potential cases will be of vital importance for the success of the recommendations. To achieve this objective, the panel suggests that the Rheumatology service reference design feedback strategies and develop awareness training sessions, periodically sending information or joint research proposals–(degree of agreement 8 3/10).
It is also essential that the Rheumatology department is ready to assume the lead. To do this, it is recommended that the waiting list be kept below a reasonable limit (ideally less than one month) (degree of agreement 8.7/10) or to establish early SpA units, as that does exist at some hospitals (level of agreement 8.8/10). In these units, the waiting time should be even lower.
At a minimum, it is recommended that SpA undergo express referral; this could include preferential referral of patients with inflammatory back pain with a positive commitment to review this “suspicion of SpA” and if possible, describing the positive signs in the examination (degree of agreement 8.3/10).
Additional RecommendationsSome panelists considered an additional advantage of PCP training in reading sacroiliac X-rays (degree of agreement 6.9/10). In general, access to care is faster in the case of AS. Moreover, in case of questionable inflammatory back pain, a true radiographic sacroiliitis, regardless of severity, can undergo a standard referral. Therefore, given that the correlation between X-ray reading done by a PCP and a rheumatologist is low,17 it is considered important to train the PCP in X-ray interpretation in the areas in which it is decided as per protocol to perform sacroiliac X-rays in PC.
In order to improve the referral, quality measurement is recommended using the following scale: (1) on rotation (or system) must indicate bypassing an SpA unit (degree of agreement 7.7/10), and (2) the time from the PC detection of the case until its evaluation for the first time in rheumatology should be less than 15 days, on average (7.6 degree of agreement/10).
DiscussionThe new classification criteria Assessment of Spondyloarthritis International Society should permit better and earlier identification of patients suffering from axial SpA, both those with radiographic evidence of sacroiliac affection (ankylosing spondylitis) as those without radiographic involvement (non radiological axial spondyloarthritis)18,19; however, this must be accompanied by a referral of patients to a rheumatologist in earlier stages. That is why we must have strategies suitable for this purpose. The main protagonists for specialist referral of patients of cases suspected of SpA are the PCP. The difficulty of making a correct derivation in cases of suspicion of SpA in patients with chronic axial pain from primary care is evident, since this type of pain is a common reason for consultation20,21 and can be related to many other causes besides16 SpA. So far, most of the guidelines, recommendations and protocols for referral of patients suspected of SpA were designed by rheumatologists, and PCP could or could not follow them depending on various circumstances.22 Since the participation in their elaboration of many hospitals and primary health care facilities in our country, and the subsequent publication of this data in the RADAR study,12 the need to design strategies for referral from PC with the participation of both rheumatologists as well as PCP became apparent.
In the expectation of seeking consensus among rheumatologists and PCP for the preparation of this document, professionals from both levels of care, who showed interest in inflammatory lumbar pathology, contributed to its development. However, we must not forget that the primary beneficiary of health is the user-patient, so this document should be submitted for the assessment of patients with SpA. Similarly, it is understood that this is a document that includes the referral processes, and must be accepted by administrators whose job it is to implant it in clinical practice, and should also be subjected to discussion among these professionals.
The final document is aimed primarily at the PCP, with criteria included for suspected cases, research algorithms and recommendations for referral to Rheumatology, and is designed to be an easy to use resource that does not disrupt consultation and consensus. The starting point is the patient with axial pain with an onset before age 45. From here, we have included key questions and extra questions about inflammatory back pain as well as other musculoskeletal manifestations, extra-articular manifestations, family history and additional tests related to the diagnosis.
In the algorithm, the importance of referral to a rheumatologist in all patients with chronic low back pain of less than 45 is emphasized on those who have features of inflammatory back pain, or signs suggestive of SpA, with HLA B27 positive or elevated CRP, or alteration of X-rays of the sacroiliac joints. The age limit of 45 years, as shown in all published algorithms, can be considered reasonable, if not entirely justified, although it can happen later in life and not always is lower back pain in those under 45 SpA. However, since the prevalence of chronic low back pain is almost double in patients over 45,21 and spondyloarthrosis is the most common cause of back pain in this group, it was felt that raising the age limit would unnecessarily increase referrals.
We want to note two limitations to these recommendations, requiring continued prudence and individualizing their application in patients. To successfully implement this protocol there must be a two-way commitment between PC and Rheumatology regarding information about the patient's condition and its final status. This would imply, however: (1) identifying the Reference area of a consultant (2) reasonable waiting times for referred patients, (3) communication on the final destination of the patient (counterreferral, if appropriate, to the PCP). In addition, some individual characteristics may act as confounding factors, particularly self-medication, which can modify some of the key questions and influence bad decision making.
In conclusion, the referral of patients under 45 years with chronic back pain from PC to Rheumatology should be done if any of these 3 possibilities exist: (1) inflammatory back pain, (2) signs suggestive of SpA (heel pain, arthritis, psoriasis, uveitis, dactylitis, inflammatory bowel disease or family history of SpA), or (3) HLA B27 positive, elevated CRP or pelvic X-ray indicative of sacroiliitis. Strategies should be designed from training and awareness by Rheumatology department to maintain optimal PCP collective collaboration in identifying cases and facilitating Rheumatology department to be prepared to take the lead.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this study research not performed experiments on humans or animals
Data confidentialityThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of InterestThe authors declare no conflict of interest.
FinancingThis project was made possible by financial and logistical support from Merck Sharp & Dohme, Spain.
Roberto Miguélez
Hernández Andrés Ariza
Juan Carlos Torre Alonso
Jesus Babio
Carolina Alvarez Castro
Mireia Martínez Moreno
Julia Fernandez
Julio Ramírez García
Sergio Rodriguez Montero
Blanca Hernandez Cruz
Fernando J. Rodríguez Martínez
Vicenç Torrente Segarra
Francisco Lorenzo Ponce
Acasuso Manuel Diaz
Rodrigo Abad Rodriguez
José Herrero Roa
Carlos Casado
Francisco Vargas Negrin
Jesus Iturralde
Mar Yague
Francisco Martínez García
Jose Francisco Sáez Martínez
Fatima Santolaya Sardinero
Carlos Gonzalez
Carlos Calvo J. Bastida
Jesus Alonso Fernández
The names of the components of the Study Group for Inflammatory Back Pain presented in Annex 1.
Please cite this article as: Juanola Roura X, Collantes Estévez E, León Vázquez F, Torres Villamor A, García Yébenes MJ, Queiro Silva R, et al. Recomendaciones para la detección, investigación y derivación del dolor lumbar inflamatorio en Atención Primaria. Reumatol Clin. 2015;11:90–98.