To develop recommendations for the use of parenteral methotrexate (MTX) in rheumatic diseases, mainly rheumatoid arthritis, based on best evidence and experience.
MethodsA group of 21 experts on parenteral MTX use was selected. The coordinator formulated 13 questions about parenteral MTX (indications, efficacy, safety and cost-effectiveness). A systematic review was conducted to answer the questions. Using this information, inclusion and exclusion criteria were established, as were the search strategies (involving Medline, EMBASE and the Cochrane Library). Three different reviewers selected the articles. Evidence tables were created. Abstracts from the European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) were evaluated. Based on this evidence, the coordinator proposed preliminary recommendations that the experts discussed and voted in a nominal group meeting. The level of evidence and grade of recommendation were established using the Oxford Center for Evidence-Based Medicine and the level of agreement with the Delphi technique (2 rounds). Agreement was established if at least 80% of the experts voted yes (yes/no).
ResultsMost of the evidence involved rheumatoid arthritis. A total of 13 preliminary recommendations on the use of parenteral MTX were proposed; 11 of them were accepted. Two of the 13 were not voted and are commented on in the main text.
ConclusionsThe manuscript aims to solve frequent questions and help in decision-making strategies when treating patients with parenteral MTX.
Desarrollar recomendaciones sobre el uso de metrotexato (MTX) parenteral en pacientes con enfermedades reumáticas, fundamentalmente en la artritis reumatoide, basadas en la mejor evidencia y experiencia.
MétodosSe seleccionó un grupo de 21 expertos reumatólogos en el manejo de MTX. El coordinador generó 13 preguntas sobre el uso de MTX parenteral (perfiles de indicación, eficacia, seguridad, costo-eficacia y biodisponibilidad) para ser contestadas mediante una revisión sistemática de la literatura. Con base en las preguntas se definieron los criterios de inclusión y exclusión, y las estrategias de búsqueda (en Medline, EMBASE y la Cochrane Library). Tres revisores seleccionaron los artículos resultantes de la búsqueda. Se generaron tablas de evidencia. Paralelamente se evaluaron abstracts de congresos de la European League Against Rheumatism (EULAR) y del American College of Rheumatology (ACR). Con toda esta evidencia el coordinador generó 13 recomendaciones preliminares que se evaluaron, discutieron y votaron en una reunión del grupo nominal con los expertos. Para cada recomendación se estableció el nivel de evidencia y grado de recomendación, y el grado de acuerdo mediante un Delphi. Se definió acuerdo si al menos el 80% de los participantes contestaron sí a la recomendación (sí o no).
ResultadosLa mayoría de la evidencia proviene de la artritis reumatoide. De las 13 recomendaciones preliminares se aceptaron 11 recomendaciones sobre el uso de MTX parenteral en reumatología. Dos no se llegaron a votar y se decidió no incluirlas, pero se comentan en el texto final.
ConclusionesEste documento pretende resolver algunos interrogantes clínicos habituales y facilitar la toma de decisiones con el uso de MTX parenteral.
Methotrexate (MTX) is an essential drug in the treatment of rheumatic diseases, especially rheumatoid arthritis (RA). Its efficacy and safety profiles are well-established,1–3 and it has been employed both in monotherapy and in combination with other agents, including biological therapies. A number of consensus statements drawn up both in Spain and by international societies have standardized its use and monitoring.4–6
However, a relatively important percentage of patients develop gastrointestinal intolerance, especially in response to increases in the dose, a fact that would limit its efficacy and even its use.7 Although it varies widely depending on the study, it has been estimated that up to 48% of the patients could have some gastrointestinal adverse event while receiving the oral formulation.8 Likewise, the rate of discontinuation due to a gastrointestinal adverse event can range from <5% to up to 16%.9 The parenteral formulation of MTX may be a good alternative in many of these cases.10 Moreover, it has been pointed out that it could prevent or delay the introduction of biological therapy, which could result in a reduction of the costs for the health system.11–13 However, there are no clear guidelines concerning the profile of the patients and of the clinical situations in which the utilization of the parenteral formulation of MTX would be beneficial.
The objective of the present article was to draft recommendations based on best evidence and experience regarding the use of parenteral MTX in patients with rheumatic diseases.
MethodsTo achieve consensus, we used the nominal group and Delphi technique.14 The preparation of the document involved the distribution of tasks and comments to the participants, with the aid of a systematic literature review and an expert methodologist.
First, we established a group of 21 experts, representing all of the geographic areas of Spain. They had demonstrated experience in the management of patients with rheumatic diseases and in the use of parenteral MTX. Their selection was based on a search in MEDLINE for Spanish rheumatologists with publications on the subject of the present document. We also took into account the submission of presentations to the meeting of the Spanish Society of Rheumatology (SER). All of the findings were discussed with the coordinator, who, in addition to the professionals referred to above, considered as experts those who usually attended specific forums, were considered to be opinion leaders concerning this issue, having presented works, etc. Finally, it was decided to make the selection ensuring the most extensive geographic representation.
Systematic Literature ReviewThe project coordinator provided 13 questions to be responded to in accordance with a systematic review (Table 1). As they all made reference to different aspects of the same subject, it was decided to conduct a single systematic literature review. These questions were used to define the inclusion and exclusion criteria. The search was for articles that included patients with any rheumatic disease who were being treated with parenteral MTX, regardless of the dose and the specific route of administration. Moreover, these reports should analyze (depending on the question) distinct aspects of the efficacy and safety of the drug treatment. Finally, the studies included had to have one of the following designs: meta-analysis, systematic review, clinical trial or observational study (prospective, retrospective and cross-sectional). The following bibliographic databases were searched: MEDLINE (from inception to January 2016), EMBASE (from inception to January 2016) and Cochrane Library (from inception to January 2016). We used MeSH terms and free text, and the search was performed with the help of an expert informationist. For each review, 3 reviewers (EL, TO, MJ) independently analyzed the articles returned in the search of the different bibliographic databases, as well as a detailed analysis of the included articles. In the case of discrepancy, it was resolved by a fourth person (LC). We reviewed the gray literature from the European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) meetings of the preceding 2 years, as well as documentation provided by the coordinator (articles and abstracts from congresses and other forums of interest related to the subject of the consensus). Subsequently, we performed a secondary hand-search of the reference lists of the articles that were to be included. To evaluate the methodological quality of the included reports, we utilized the Jadad scale for randomized controlled trials (RCT) and that of the Oxford Center for Evidence-Based Medicine for the remaining designs.15 We created evidence tables and tables showing the results that describe the main characteristics and findings of the included studies.
Questions Generated by the Coordinator the Responses to Which Were Encountered in the Systematic Literature Review.
| Number | Question |
|---|---|
| 1 | What is the difference in terms of bioavailability of MTX depending on whether it is administered orally or parenterally? |
| 2 | What are the differences in the clinical efficacy of MTX depending on whether it is administered orally or parenterally? |
| 3 | Does the safety and tolerability of MTX improve when administered by the parenteral route? |
| 4 | What finding in a patient profile should lead a physician to recommend the initiation of parenteral administration of MTX therapy and when? |
| 5 | When should the parenteral route be employed? What is the starting dose? Is it recommendable to increase or reduce it? |
| 6 | What is the maximum dose of parenteral MTX that should be administered before the treatment can be considered ineffective? |
| 7 | When MTX is combined with a biological agent, what utility is provided by the parenteral route of administration of the drug? |
| 8 | What is the equivalence between oral and parenteral doses? |
| 9 | What is the impact of the parenteral route for MTX administration from the point of view of cost-effectiveness? |
| 10 | What impact does the parenteral route have on adherence? |
| 11 | What impact does the parenteral route of MTX administration have on patient quality of life (as compared to the oral route)? |
| 12 | What advantages are offered by the concentrated solution (50mg/mL) of MTX for parenteral administration |
| 13 | What is the impact of educating the patient on self-administration of parenteral MTX therapy? |
mg, milligram; mL, milliliter; MTX, methotrexate.
The group of experts held a nominal group meeting. Prior to the meeting, the results of the systematic literature review were sent to all the participants, together with the preliminary recommendations drawn up for each question on the basis of the evidence encountered. During the meeting, the recommendations and the above evidence were presented and discussed.
After that, the definitive recommendations were formulated and were rated according to the Delphi technique, utilizing a system of electronic voting. Agreement was considered to be reached if at least 80% of the participants responded “yes” to the recommendation (yes or no). The results of the Delphi process were shown to the experts. The recommendations with a level of agreement (LA) of less than 80% were reevaluated and, if necessary, were rewritten and voted again in a second round.
Final Consensus DocumentThe final document was based on all of this information. Each of the recommendations, with the aid of the methodologist, were assigned a level of evidence (LE) and a grade of recommendation (GR), in accordance with the recommendations for evidence-based medicine of the Oxford Center for Evidence-Based Medicine.15 The assignment of the LA was carried out as was reported above. The document was circulated among the experts for their final evaluation and concluding comments.
Throughout the document, we speak of parenteral MTX in generic terms, that is, including the subcutaneous (SC) and intramuscular (IM) routes (and, exceptionally, the intravenous [IV] route). However, on the basis of reported studies, in general, this term is applied to the SC route, that which is most widely utilized in clinical practice. In those points in the text that required (according to the panelists) a specification of the route, this was provided.
ResultsThe search identified a total of 1884 articles, 164 of them were duplicates. In all, 1720 references were evaluated by title and abstract, and there were 64 in which the full text was examined. This step eliminated 26, most of them because of their design or the fact that they did not present specific data on MTX. The systematic literature review ultimately included 38 articles, the majority dealing with patients with RA. Of the 13 preliminary recommendations, 9 were accepted to be voted on and generated 2 additional ones (Table 2). Here we describe the 11 recommendations that were finally accepted and the available evidence concerning each question.
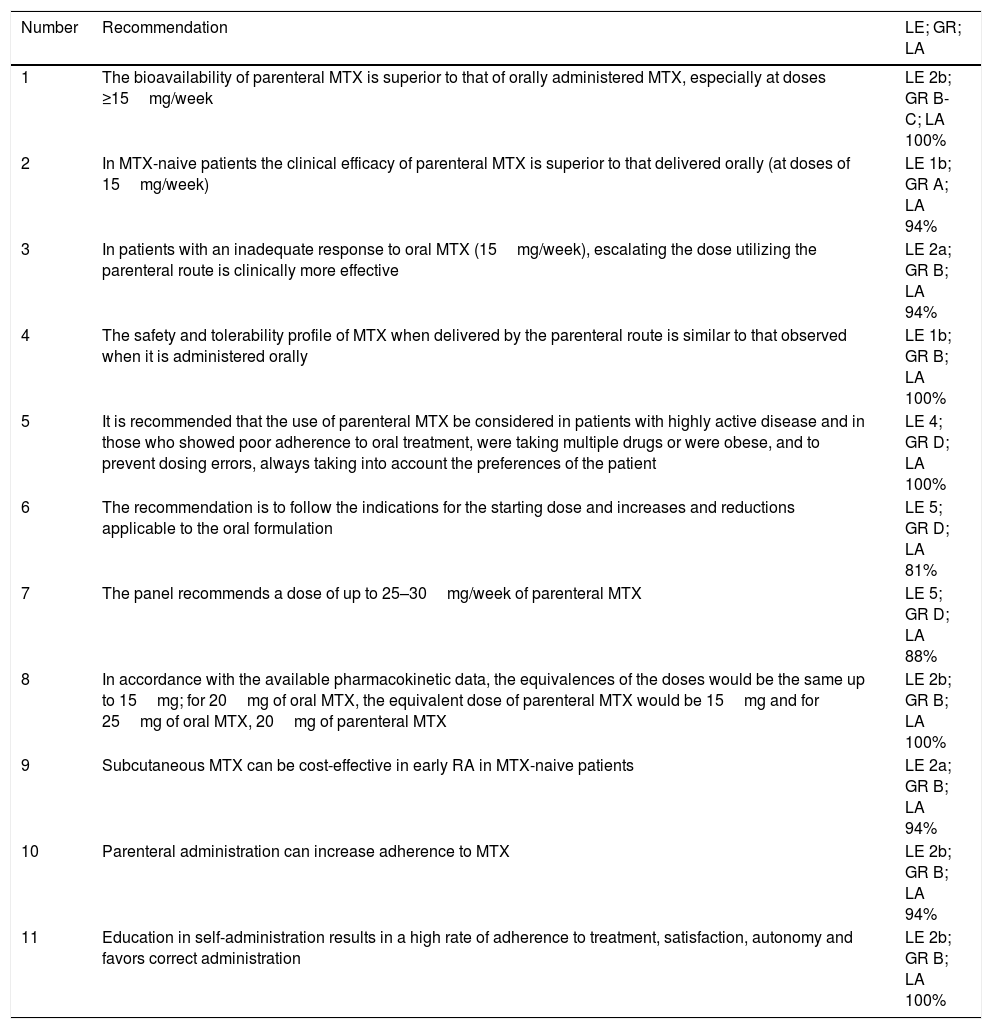
Recommendations With Level of Evidence, Grade of Recommendation and Level of Agreement.
| Number | Recommendation | LE; GR; LA |
|---|---|---|
| 1 | The bioavailability of parenteral MTX is superior to that of orally administered MTX, especially at doses ≥15mg/week | LE 2b; GR B-C; LA 100% |
| 2 | In MTX-naive patients the clinical efficacy of parenteral MTX is superior to that delivered orally (at doses of 15mg/week) | LE 1b; GR A; LA 94% |
| 3 | In patients with an inadequate response to oral MTX (15mg/week), escalating the dose utilizing the parenteral route is clinically more effective | LE 2a; GR B; LA 94% |
| 4 | The safety and tolerability profile of MTX when delivered by the parenteral route is similar to that observed when it is administered orally | LE 1b; GR B; LA 100% |
| 5 | It is recommended that the use of parenteral MTX be considered in patients with highly active disease and in those who showed poor adherence to oral treatment, were taking multiple drugs or were obese, and to prevent dosing errors, always taking into account the preferences of the patient | LE 4; GR D; LA 100% |
| 6 | The recommendation is to follow the indications for the starting dose and increases and reductions applicable to the oral formulation | LE 5; GR D; LA 81% |
| 7 | The panel recommends a dose of up to 25–30mg/week of parenteral MTX | LE 5; GR D; LA 88% |
| 8 | In accordance with the available pharmacokinetic data, the equivalences of the doses would be the same up to 15mg; for 20mg of oral MTX, the equivalent dose of parenteral MTX would be 15mg and for 25mg of oral MTX, 20mg of parenteral MTX | LE 2b; GR B; LA 100% |
| 9 | Subcutaneous MTX can be cost-effective in early RA in MTX-naive patients | LE 2a; GR B; LA 94% |
| 10 | Parenteral administration can increase adherence to MTX | LE 2b; GR B; LA 94% |
| 11 | Education in self-administration results in a high rate of adherence to treatment, satisfaction, autonomy and favors correct administration | LE 2b; GR B; LA 100% |
GR, grade of recommendation; LA, level of agreement; LE, level of evidence; mg, milligram; MTX, methotrexate; RA, rheumatoid arthritis.
The bioavailability of parenteral MTX is superior to that of orally administered MTX, especially at doses ≥15mg/week (LE 2b; GR B-C; LA 100%).
Several studies (moderate-to-low quality, Oxford 2a-4) assessing the pharmacokinetics over a limited time period,16–22 show that increasing the dose of oral MTX reduces its bioavailability, which reaches its plateau at 15mg/week, a phenomenon that is not observed with parenteral MTX (positive relationship of the area under the curve on increasing the dose of parenteral MTX). That is, starting at 15mg/week, the bioavailability of parenteral MTX will be superior to that of oral MTX, while they are similar at lower doses.
What Are the Differences in the Clinical Efficacy of Methotrexate Depending on Whether It Is Administered Orally or Parenterally?Recommendation 2In MTX-naive patients the clinical efficacy of parenteral MTX is superior to that delivered orally (at doses of 15mg/week) (LE 1b; GR A; LA 94%).
Recommendation 3In patients with an inadequate response to oral MTX (15mg/week), escalation of the dose utilizing the parenteral route is clinically more effective (LE 2a; GR B; LA 94%).
A RCT23 involving RA patients who were naive to MTX, many with early RA, compared the efficacy of oral MTX with parenteral MTX at the same doses (15mg/week) for 6 months (good quality, Oxford 1b). The authors observed superiority (statistically significant) of the parenteral route in the ACR criteria for 20% and 70% improvement, that is, ACR20 and ACR70 responses and in the reduction of the number of swollen joints, with no differences in the ACR50 response or changes in the Health Assessment Questionnaire. On the other hand, 30% of the subgroup of patients who failed to respond to the oral formulation and switched to the parenteral route in week 16 achieved ACR20 6 months later.
In another RCT24 with a duration of 6 months (Oxford 2c), parenteral MTX was significantly superior to oral MTX in the rates of ACR20 and ACR50 responses, among other findings. There were no differences in the ACR70 response, pain severity, patient global assessment or morning stiffness.
Observational studies of low-to-moderate quality have shown that patients who switch from oral MTX (due to lack of efficacy or toxicity) to parenteral MTX can achieve a satisfactory therapeutic response, especially over the short term.25–28
Does the Safety and Tolerability of Methotrexate Improve When Administered by the Parenteral Route?Recommendation 4The safety and tolerability profile of MTX when delivered by the parenteral route is similar to that observed when it is administered orally (LE 1b; GR B; LA 100%).
In general, both in RCT23,24 and in observational studies, parenteral MTX does not increase the rate, type or severity of the adverse events reported with the oral formulation.9,25,28–34 The rates of discontinuation or interruption of parenteral MTX are reported to be variable. Up to 1 year of follow-up, the rate of discontinuation ranged from 0% to 18%,9,25,3134,35 depending on the study, and, in an article with long-term results, the rate after 2 years of follow-up was 25% and, at 5 years, was 53%.35
However, the heterogeneity in terms of defining and coding adverse events suggests that we should interpret these data with caution.
It has been pointed out that parenteral MTX administration could reduce the rate of gastrointestinal adverse events associated with oral MTX. The findings in RCT are contradictory.23,24 A low-quality observational study specifically compared the severity of gastrointestinal adverse events occurring with the 2 formulations36 and demonstrated that, in general (although it depended on the type of adverse event), the severity was greater with the oral formulation.
What Finding in a Patient Profile Should Lead a Physician to Recommend the Initiation of Parenteral Administration of Methotrexate Therapy and When?Recommendation 5It is recommended that the use of parenteral MTX be considered in patients with highly active disease and in those who showed poor adherence to oral treatment, were taking multiple drugs or were obese, and to prevent dosing errors, always taking into account the preferences of the patient (LE 4; GR D; LA 100%).
Parenteral MTX has been shown to be effective in different patient profiles, including MTX-naive patients (with or without previous disease-modifying antirheumatic drugs [DMARDs])32,37,38 and in biologic-naive patients,23,38,39 as well as in patients who did not respond to oral MTX because of its lack of efficacy or its toxicity.13,25,40,41
Moreover, there is indirect evidence concerning certain profiles. For example, in patients with poor adherence to oral therapy, the use of prefilled syringes with previous training achieves very high rates of adherence and satisfaction.33 Thus, it would be feasible to consider that parenteral MTX can improve adherence in patients whose compliance with oral treatment is poor. This same principle could be applied to patients taking multiple drugs and/or with comorbidities (avoiding oral medication), or who are overweight or obese (because of its greater bioavailability). Patient preferences, the prevention of dosing errors and economic questions concerning the health system (to avoid or delay the initiation of biological therapy) should also be taken into account.
When Should the Parenteral Route Be Employed? What Is the Starting Dose? Is It Recommendable to Increase or Reduce It?Recommendation 6The recommendation is to follow the same indications for the starting dose and increases and reductions applicable to the oral formulation (LE 5; GR D; LA 81%).
No starting dose or dose escalation has been shown to be clearly superior to others. In a RCT involving MTX-naive patients, parenteral MTX was begun at a dose of 15mg/week,23 and in another good-quality RCT (Oxford 1b)42 the starting dose was 7.5mg/week for 2 weeks and was then escalated to 15mg/week. Thus, the panel recommends to start with a dose of 15mg/week in MTX-naive patients. In observational studies, the starting dose in MTX-naive patients varies widely from 5mg/week to doses of ≥25mg/week.19,31,43
In patients who do not respond to oral MTX, RCT report diverse starting doses; at times, it is the same dose reached with the oral formulation,44 and others follow a scheme employing prefilled doses,24,39,45 although the majority choose the parenteral route, initially at doses of at least 15mg/week. Some observational studies start with the parenteral route at a dose utilized for oral delivery; on other occasions, the authors follow a predetermined scheme.19,31,43 Thus, the recommendation of the panel in the case of patients who switch from the oral route, is that they begin with the same dose, taking into account that, depending on the amount, it will be necessary to carry out a conversion to calculate the parenteral dose. Likewise, it is suggested that lower doses of parenteral MTX than those recommended be considered for certain types of patients or circumstances such as elderly individuals, those with a low body mass index, those with chronic kidney disease, etc.
With regard to dose escalation, some authors propose increases of 2.5mg/every 2 weeks or 5mg/month; others, follow the steps of a predetermined scheme, and there are articles in which escalations depend on the clinical response.23–25,38
Finally, with respect to de-escalation, it is mentioned very little in the literature. It is carried out on the basis of the clinical outcome once the therapeutic objective has been achieved.43
What Is the Maximum Dose of Parenteral Methotrexate That Should Be Administered Before the Treatment Can Be Considered Ineffective?Recommendation 7The panel recommends a dose of up to 25–30mg/week of parenteral MTX (LE 5; GR D; LA 88%).
The maximum reported dose varies depending on the study and the route of administration; for example, it reaches 10–45mg with the IM route, 10–30mg using the SC route and up to 50mg with the IV formulation. The maximum dose of parenteral MTX most widely employed (due to its frequency) is around 20–25mg/week.19,23–25,31,38,43
On the other hand, there is no clear maximum dose limited by toxicity, lack of clinical efficacy or tolerance phenomena.
When Methotrexate Is Combined With a Biological Agent, What Utility Is Provided by the Parenteral Route of Administration of the Drug?We do not have sufficient evidence to respond to that question at the present time.
A 2015 oral presentation from the ACR46 reported that, in a cohort of nearly 20,000 RA patients, survival at 12 months of those receiving the combination of the biological plus MTX, was very similar when the parenteral and oral formulations were compared (47% vs 44%).
In a retrospective observational study32 involving 70 patients with early RA who were naive to all DMARDs (synthetic and biological). They were divided into 2 groups—parenteral MTX and parenteral MTX plus a biological agent. No statistically significant differences were observed between the groups in terms of Disease Activity Score in 28 joints (DAS28), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), time to remission, low RA activity, use of corticosteroids or rates of discontinuation. However, in these patients, there were differences in the comparison of DAS28, ESR and CRP before and after the addition of the biologic (in favor of the combination).
Finally, in relation to the indication for biological therapies, some authors13,31 point out that parenteral MTX can delay or avoid the introduction of a biological agent.
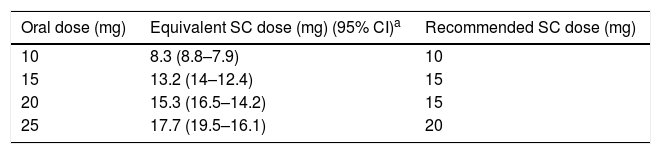
What Is the Equivalence Between Oral and Parenteral Doses?Recommendation 8In accordance with the available pharmacokinetic data, the equivalences of the oral and parenteral doses would be the same up to 15mg; for 20mg of oral MTX, the equivalent dose of parenteral MTX would be 15mg and for 25mg of oral MTX, 20mg of parenteral MTX (LE 2b; GR B; LA 100%).
The majority of published studies utilize the same dose as the oral dosage or follow a predetermined scheme for the switch. However, there are 2 studies that, although in an intuitive manner, propose a conversion. One is a RCT42 in which oral MTX is changed to the IM route, assuming a mean bioavailability of 70%; thus, 15mg IM MTX would be 21.4mg of oral MTX. The other is a moderate-to-low quality observational study18 (Oxford 3b), which likewise, considered a bioavailability of 2/3 of the parenteral route.
More recently, on the basis of a study on bioavailability (pharmacokinetics),47 the authors propose a dose conversion table that is recommended by the panel (see Table 3).
Methotrexate Bioequivalence.
| Oral dose (mg) | Equivalent SC dose (mg) (95% CI)a | Recommended SC dose (mg) |
|---|---|---|
| 10 | 8.3 (8.8–7.9) | 10 |
| 15 | 13.2 (14–12.4) | 15 |
| 20 | 15.3 (16.5–14.2) | 15 |
| 25 | 17.7 (19.5–16.1) | 20 |
CI, confidence interval; mg, milligram; SC, subcutaneous.
Subcutaneous MTX can be cost-effective in early RA in MTX-naive patients (LE 2a; GR B; LA 94%).
A study of quality and the cost-effectiveness of parenteral MTX in MTX-naive RA patients adjusted according to the circumstances (including costs) of the Spanish health system,48 observed a cost/quality-adjusted life year (pharmacologic) at 5 years of €25,173–€35,807, which was €19,056–€25,351 for patient lifetime.
On the other hand, in cost-minimization studies,11,12 it has been seen, for example, that utilizing the costs in the United Kingdom, the use of parenteral MTX in patients having an insufficient response to oral MTX can save £7197 per patient in the first year and £9.3 million per year in new patients, by delaying the introduction of biological therapies.
Other studies,13 on the basis of their results, have calculated that for every 1000 RA patients there are 40 receiving parenteral MTX and, taking into account that in 36 of these individuals, the response is equivalent to that achieved with an anti-tumor necrosis factor agent, the savings (by not administering a biological therapy) would be £306,000 or, what is the same, £300 per patient-year.
What Impact Does the Parenteral Route Have on Adherence?Recommendation 10Parenteral administration can increase adherence to MTX (LE 2b; GR B; LA 94%).
One study (of good-to-moderate quality, Oxford 2b) evaluated adherence to parenteral drugs in 40 RA patients with psoriatic arthritis, 20 of whom began with parenteral MTX. All of them received education and training on self-injection.29 There was an adherence to the treatment ≥80% in 92.5% of the patients. In another study49 of moderate-to-low quality (Oxford 3b) involving 12 patients with rheumatic diseases who switched from the oral route to IM administration. At 6 months, there were no differences in adherence, as they had interrupted MTX due to the lack of efficacy or toxicity. In another report,28 after analyzing 30 RA patients who switched from oral to parenteral MTX, at 6 months, there was only one patient who did not adhere to the treatment.
On the other hand, a number of studies evaluated the effect of self-administration with a prefilled syringe. Many of the patients receiving training in self-administration,29,30,33,38,39 and, over the short term, the authors observed a high adherence to the treatment.
Moreover, a systematic review that analyzed survival or discontinuation of parenteral MTX9,49 found that maintenance of the drug decreases over time, and that survival of the parenteral drug was superior to oral MTX.
With respect to parenteral MTX, there is also a concentrated solution (50mg/mL) that, over the short term,33,38 has been found to be preferred both by patients and by health professionals because its administration is associated with fewer errors and with greater independence, quality of life and patient satisfaction.
Finally, it is important to take into account the role that education and training can have in self-administration in the rate of adherence, as do the proposed needles and the lower prefilled volume.29,33,38
What Impact Does the Parenteral Route of Methotrexate Administration Have on Patient Quality of Life (as Compared to the Oral Route)?We do not have sufficient evidence to respond to that question at the present time.
A high-quality RCT, with a duration of 22 weeks, evaluated quality of life employing Short Form 12 (SF-12).45 In this study, the authors compared the use of IM MTX applying escalation at a fixed dose of 15mg/week, and found no differences between the groups.
Again, indirectly, and taking into account the high level of satisfaction and preference associated with the parenteral formulation, this can contribute to improving the quality of life of patients.29,33,38
What Is the Impact of Educating the Patient on Self-administration of Parenteral Methotrexate Therapy?Recommendation 11Education in self-administration results in a high rate of adherence to treatment, patient satisfaction and autonomy, and favors correct administration (LE 2b; GR B; LA 100%).
A number of studies29,30,33,38,39 of varying quality have shown that, at least over the short term, education in self-administration results in a high rate of adherence to the treatment, considerable patient satisfaction, independence, improvement in quality of life and good local tolerance. This, in turn, can have an impact on a more satisfactory control of the patient.
Finally, we wish to point out that, in terms of education, there is a wide variation in the form, format and content. In general, the nursing staff is in charge of instructing patients. Moreover, in certain training programs, materials are provided on paper.
DiscussionIn the present document, we present a series of recommendations on the use of parenteral MTX based on the best evidence currently available, mostly associated with RA.
Methotrexate is a standard drug in the treatment of RA. After the disease has been diagnosed, its indication as the first line of treatment, and as early as possible, is clearly defined, as is strongly recommended by different societies4–6; it is suitable as an anchor drug for combined therapies.
However, there continues to be a wide variation in clinical practice in Spain with respect to the starting dose, the rate and pattern of escalation of this dose, selection of routes of administration and dosing with concomitant use of folic acid or folinic acid in RA patients.
In the literature, different international groups have formulated recommendations on the use of MTX in general, but mostly refer to the oral formulation. As is the case with the present document, all of them consider that the parenteral option is optimal in patients with intolerance or an inadequate clinical response to the oral formulation.50–52
Although the strategy of searching and reviewing the gray literature was extensive, the major limitation to the recommendations is the quality of the studies that propose them, which, aside from exceptions, is moderate-to-low. However, MTX is a drug that has been utilized for many years in rheumatology. Thus, there is a great deal of experience in its use. In the present document, the experts were very much in agreement with the wording of the recommendations. Another important limitation is the fact that, although they exist, there are few RCT that compare formulations and long-term comparative studies are practically nonexistent. Moreover, nearly all of the evidence refers to RA, which makes it difficult to extrapolate it to other rheumatic diseases.
On the other hand, there are still questions to be resolved concerning the use of parenteral MTX. One of the most important of them is the dosage (starting dose, maximum dose and escalation). At the present time, the recommendations established for the oral formulation continue to be followed. We also need studies that evaluate the efficacy of the parenteral formulation in terms of patient quality of life and its role in combination with biological therapies.
In terms of improving clinical practice, it is essential to establish explicit recommendations that comprise aspects as important as the indication or the bioequivalence of parenteral MTX with respect to the oral formulation. Although there is still little evidence in some areas of this issue, this document presents a series of recommendations that we consider to be relevant and useful for clinicians, that complement those mentioned above concerning the general use of MTX.4 Moreover, they refer to routine aspects related to its use, which means that the recommendations can be implemented without difficulties in daily practice. The panel is convinced that following them will improve patient management and, thus, the prognosis.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FinancingThe project was financed by Gebro Pharma, which did not intervene in the design of the project or in the drafting of the recommendations.
Conflicts of InterestThe authors received remuneration from Gebro Pharma for their role in developing the project.
We thank Ms. Estibaliz Loza for her invaluable help and support in the preparation of this document.
Please cite this article as: Tornero Molina J, Calvo Alen J, Ballina J, Belmonte MÁ, Blanco FJ, Caracuel MÁ, et al. Recomendaciones sobre el uso de metrotexato parenteral en enfermedades reumáticas. Reumatol Clin. 2018;14:142–149.