To analyze the frequency and characteristics of dose reduction of biological agents in a cohort of patients with chronic arthritis, in clinical practice conditions in a tertiary level hospital.
Materials and methodsDescriptive, cross-sectional study, which included all patients, followed consecutively during 6 months (June 2011–November 2011), by one investigator, with patients who at least have received one dose of biological agents in 2011.
ResultsWe included 153 patients: rheumatoid arthritis (RA) (n=82), ankylosing spondylitis (n=29), psoriatic arthritis (n=20), and miscellaneous group (n=22). Mean disease duration was 14.9±7.7 years. At the time of analysis, 70 patients (45.7%) were receiving low doses of biological therapy (50% in miscellaneous group group, 50% in psoriatic arthritis, 48.2% in ankylosing spondylitis, and 42.6% in RA). Mean time of dosage reduction was 17.4±17.5 months. The most common biological agents used in low dose were: etanercept, adalimumab and tocilizumab in 57.6%, 54.9%, and 40% respectively, in patients with a reduced dose of biological therapy. The patients at low dose of biological therapy compared with standard dose had similar mean disease duration, but received significantly less DMARDs, glucocorticoids and NSAIDs, and similar biological agent duration. RA patients with reduced biological treatment, at the time of analysis, had higher remission rates versus patients receiving a standard dose (82.9% vs 34%, P<.0001). The medical decision at the time of analysis was to maintain low-dosage biological treatment in almost all patients.
ConclusionIn our clinical practice, 45.7% of our chronic arthritis patients receive low dose of biological therapy, after achieving remission or low activity at standard doses, maintaining a good control of the disease.
Analizar la frecuencia y características de la reducción de dosis de fármacos biológicos en una cohorte de pacientes con artritis crónica, en condiciones de práctica clínica de un hospital de tercer nivel.
Material y métodosEstudio descriptivo y transversal, que incluyó a todos los pacientes visitados consecutivamente durante 6 meses (junio de 2011-noviembre de 2011) por un solo investigador, con pacientes que habían recibido al menos una dosis de fármaco biológico durante el año 2011.
ResultadosSe incluyeron 153 pacientes: artritis reumatoide (AR) (n=82), espondilitis anquilosante (n=29), artritis psoriásica (n=20) y grupo miscelánea (n=22) con una evolución media de 14,9 ± 7,7 años. En el momento del análisis, 70 pacientes (45,7%) estaba con dosis reducida (un 50% en el grupo miscelánea; un 50% en artritis psoriásica; un 48,2% en espondilitis anquilosante, y un 42,6% en AR). El tiempo medio de reducción de dosis fue de 17,4 ± 17,5 meses. Los fármacos biológicos más utilizados a dosis reducidas fueron: etanercept, adalimumab y tocilizumab; el 57,6, el 54,9 y el 40 respectivamente de los pacientes tratados con estos agentes lo hacían a dosis reducidas. Los pacientes con dosis reducidas en comparación con aquellos con dosis normales tenían un mismo tiempo de evolución de la enfermedad, pero recibían menos FAME, glucocorticoides y AINE, con un tiempo similar de uso del agente biológico. Los pacientes con AR y dosis reducidas tenían, en el momento del análisis, mayores índices de remisión que los pacientes con dosis normales (82,9 vs. 34%, p < 0,0001). La decisión terapéutica en el momento del análisis fue mantener la dosis reducida en la práctica totalidad de los pacientes.
ConclusiónEn nuestra práctica clínica, el 45,7% de los pacientes con artritis crónica reciben terapia biológica a dosis reducidas, tras haber alcanzado la remisión o baja actividad a dosis estándares, manteniendo la mayoría de ellos un buen control de la enfermedad.
Biological therapies are one of the most important advances in the treatment of various types of chronic arthritis and immune-mediated processes in recent decades. Their clinical efficacy has been amply confirmed in I clinical trials and observational studies with an acceptable safety and tolerability profile.1 Biological therapies include TNF antagonists (infliximab, etanercept, adalimumab, golimumab and certolizumab) all with indications in rheumatoid arthritis (RA) and, except certolizumab, also approved for use in psoriatic arthritis (PsA) and ankylosing spondylitis (AS). In RA non-anti-TNF biologicals have also received approval, such as abatacept, rituximab and tocilizumab. There are virtually no head to head studies between the various biological drugs, but several meta-analyzes and systematic reviews show similar efficacy between them although some differences exist regarding their safety profile.2,3
With the introduction of these biological agents, many patients who have been deemed DMARD refractory, as in the case of RA or psoriatic arthritis, or NSAIDs in the case of AS, respond favorably and in a significant number there is a very good response, even achieving a state of remission or low activity.4,5 These patients are initially treated with doses recommended by the data sheet according to the results of phase iii clinical trials for product registration and approval by competent authorities, but there is no consensus on what attitude to take in presence of sustained remission. There are data in the literature that show that the abandonment of these therapies produces a clinical relapse of the disease in the majority of cases.6,7 However, some observational studies8 and recommendations by management guidelines for inflammatory arthritis such as RA9 suggest the possibility of reducing the dose of biological agents in these patients to the lowest effective dose, with the idea that some patients, perhaps treated too intensively with standard doses, could get the same benefit with a lower dose.10 This dose reduction practice has been implemented empirically in recent years in the rheumatology practice, partly to achieve cost reductions at a time of economic crisis.
In this cross-sectional study, we present our experience with dose reduction in a group of patients with rheumatic diseases in clinical practice conditions.
Materials and MethodsThis was designed as a descriptive, cross-sectional and retrospective study, which included some patients consecutively and for 6 months (from June 2011 to November 2011), all from an outpatient Arthritis Unit, Rheumatology Department of a specialty hospital, seen by a single investigator, and who had received at least one dose of biological agent in 2011. All the patients were treated according to the management guidelines of the Spanish Society of Rheumatology (SER) as per diagnosis. The objective of the study was to analyze how many patients were receiving a reduced dose of biological agent at the time of analysis, study their characteristics and compare them to the group of patients who continued with a standard dose. A “low-dose” was defined as a treatment regimen with a reduced amount of drug than that recommended in the data sheet for each product, either by using lower doses or by increasing intervals between dose administration.
The reduced dose regimen was established empirically, on the basis of a suitable control and maintenance of a level of the activity of the disease (in most cases remission according to activity rates and for ≥12 months) performed by the rheumatologist who assessed patients in clinical practice conditions, although not using any strategy or default protocol.
The patients in whom the drug was suspended due to adverse events were removed from the study.
Each of the patients included came for a check-up visit, and data collection at the time of the analysis was performed and reviewed, retrospectively, through clinical history data.
We analyzed the following variables: demographics (gender and age), diagnosis years of evolution of the disease, previous treatment (synthetic and biological DMARDs), current biological treatment (type, dose and duration of biological therapy) and current and concomitant treatment (synthetic DMARDs and/or corticosteroids). In patients diagnosed with RA we also determined serum ESR and CRP at the time of inclusion; and calculated the compound clinical activity index DAS28 at the time of analysis.11
In those patients receiving reduced biological doses at the time of analysis we studied: the reason for dose reduction, reduced time-dose and clinical decision at the time of analysis (maintenance or biological agent at a reduced dose). In patients receiving standard biological doses at the time of inclusion we analyzed whether such a reduction had been previously tried. We also examined whether there were differences in the variables included in both groups (low dose vs normal dose).
Statistical AnalysisA descriptive study was conducted using measures of central tendency (mean) and dispersion (standard deviation) for continuous variables, and percentages for qualitative variables. We performed a comparative analysis of the 2 groups of patients (reduced dose vs normal dose) using the Chi-square test for qualitative variables, and Student's t-for quantitative variables. Significance was set at P<.05.
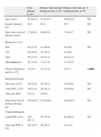
ResultsFrom June 2011 to November 2011 a total of 169 patients were initially included of whom some were excluded for not receiving the biological agent at the time of assessment (n=2) or because the treatment duration was less than 6 months (n=14). Of the 153 patients included in the final analysis, 63.3% were women and the average age was 51 years. The main diagnosis was RA in 53.5% of patients. Other diagnoses were: EA (18.5%), PsA (13.5%) and miscellaneous (14.5%), including 8 juvenile idiopathic arthritis, 3 undifferentiated spondyloarthropathy, 3 seronegative polyarthritis, 3 uveitis, 2 palindromic rheumatism, one mixed connectivetissue disease and one case of SAPHO syndrome and Still's disease. Baseline characteristics of all patients examined, as well as patients per group (low and standard dose) are shown in Table 1. At the time of analysis, 70 patients (45.7%) received a reduced dose of biological either due to a decrease in dosage or an increase of the administration interval. In none of the patients with reduced doses was a dose decreased due to adverse effects.
Baseline Characteristics of the Total Patient and Comparison Groups at the Time of Analysis.
| Total patients, n=153 | Patients with standard biological dose, n=83 | Patients with reduced biological dose, n=70 | P | |
| Age (years) | 51.0±14.3 | 52.1±13.4 | 49.8±15.3 | NS |
| Gender (female) (%) | 63.3 | 63.8 | 55.7 | NS |
| Time since onset of disease (years) | 7.7±14.9 | 8.0±15.0 | 7.3±14.7 | NS |
| Diagnosis, n (%) | ||||
| RA | 82 (53.5) | 47 (56.6) | 35 (50) | NS |
| SA | 29 (18.5) | 15 (18.1) | 14 (20) | |
| PsA | 20 (13.5) | 10 (12.1) | 10 (14.3) | |
| Miscellaneousa | 22 (14.5) | 11 (13.2) | 11 (15.7) | |
| ≥2 prior biological agents, n (%) | 14 (9.1) | 11 (13.2) | 4 (5.7) | <.0001 |
| Biological therapy | ||||
| Previous, n (%) | 39 (25.5) | 25 (30.1) | 14 (20.0) | NS |
| Anti-TNF, n (%) | 34 (22.2) | 20 (24.1) | 14 (20.0) | NS |
| Non-anti TNF | 5 (3.3) | 5 (6.0) | – | |
| Time from start of biologic therapy (m) | 55.1±34.3 | 55.3±38.0 | 54.9±29.5 | NS |
| Current biological | ||||
| Anti-TNF, n (%) | 125 (81.7) | 63 (75.9) | 62 (88.6) | NS |
| Non anti-TNF, n (%) | 28 (18.3) | 20 (24.1) | 8 (11.4) | |
PsA: psoriatic arthritis, RA: rheumatoid arthritis, SA: ankylosing spondylitis, m: months, n: number, NS: not significant, TNF: tumor necrosis factor.
In bold, statistically significant data.
25.5% of patients had previously received other biologic drugs, with this circumstance being less frequent in the group of patients with reduced doses than in patients with standard doses, although these differences did not reach statistical significance. No differences were seen in the duration of the current biological therapy in both groups (Table 1). In most patients, current biological therapy includes the anti-TNF drugs, with no significant difference between the 2 groups. In the group of patients with reduced dose, the average time for such a reduction was 17.4±17.5 months.
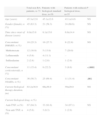
When reduced dose patients were analyzed with respect to the biologic drug used, 57.6% of those receiving adalimumab were receiving a reduced dose, 54.9% of those taking etanercept, 40% of those receiving tocilizumab and 14.3% of patients treated with infliximab. No dose reduction was seen in patients treated with other biologics. The most employed reduced dose was 50mg/10 days etanercept (48.7% of patients with reduced dose of etanercept), 40mg/21 days for adalimumab (78.9%), 5mg/kg/9 weeks for infliximab (50%) and 6mg/kg/4 weeks for tocilizumab (87.5%) (Table 2). When we analyzed the group of patients with a reduced dose with regard to the underlying disease, 50% of those in the miscellaneous group had a reduced dose, as well as 50% of PsA, 48.2% of patients with AS, and 42.6% of RA. 24 of the 83 patients receiving a standard dose (28.9%) had previously tried to reduce biologic drug dose without success, having to return to the normal dose.
Patients With Reduced Doses: Drugs and Doses Used.
| Patients with reduced biological dose (n=70), n (%) | |
| Infliximab | 4 (5.7) |
| 5mg/9 weeks | 2 (2.9) |
| 5mg/10 weeks | 1 (1.4) |
| Othera | 1 (1.4) |
| Etanercept | 39 (55.7) |
| 50mg/10 days | 19 (27.1) |
| 50mg/15 days | 12 (17.1) |
| 50mg/21 days | 3 (4.3) |
| 25mg/7 | 2 (2.9) |
| Other | 3 (4.3) |
| Adalimumab | 19 (27.1) |
| 40mg/21 days | 15 (21.4) |
| 40mg/30 days | 4 (5.7) |
| Tocilizumab | 8 (11.4) |
| 6mg/kg/4 weeks | 7 (10) |
| 4mg/kg/4 weeks | 1 (1.4) |
n: number, %: percentage of the total.
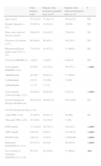
In patients diagnosed with RA no significant differences were seen in demographic characteristics, duration of illness and duration of current biological therapy between the standard-dose group and low-dose group, although in patients with a reduced dose the frequency of use NSAIDs and corticosteroids was significantly lower (Table 3).
Characteristics of the 71 Patients With a Diagnosis Other Than Rheumatoid Arthritis Undergoing Biologic Therapy at Standard or Reduced Doses.
| Total non RA patients, n=71 | Patients with biological standard dose, n=36 | Patients with reduced biological dose, n=35 | P | |
| Age (years) | 45.3±12.8 | 45.1±12.6 | 43.1±14.6 | NS |
| Gender (female), n (%) | 45 (63.3) | 21 (58.3) | 24 (68.6) | NS |
| Time since onset of disease (years) | 8.0±15.0 | 8.1±15.0 | 6.9±14.4 | NS |
| Concomitant DMARD, n (%) | 18 (25.3) | 10 (27.7) | 8 (22.8) | NS |
| Methotrexate | 12 (16.9) | 5 (13.9) | 7 (20.0) | |
| Leflunomide | 4 (5.6) | 4 (11.1) | – | |
| Sulfasalazine | 2 (2.8) | 1 (2.8) | 1 (2.9) | |
| Concomitant corticosteroids, n (%) | 11 (15.4) | 8 (22.2) | 3 (8.6) | <.0001 |
| Concomitant NSAIDs, n (%) | 36 (50.7) | 25 (69.4) | 11 (31.4) | .001 |
| Current biological therapy duration, (m) | 63.2±30.9 | 66±36.9 | 59±29.0 | NS |
| Current biological drug, n (%) | ||||
| Anti-TNF, n (%) | 67 (94.3) | 33 (91.6) | 34 (97.1) | NS |
| Non anti TNF, n (%) | 4 (5.6) | 3 (8.3) | 1 (2.9) | |
NSAIDs: non-steroidal anti-inflammatory drugs, DMARDs: disease-modifying drug, m: months, n: number, NS: not significant, TNF: tumor necrosis factor.
In bold, statistically significant data.
In RA patients there were no significant differences between patients with reduced doses and standard dose patients, taking into account with patients with reduced dose having less concomitant use of DMARD and glucocorticoid compared to patients with standard doses (Table 4). No differences were observed between groups regarding the duration of the disease. When retrospectively analyzed, values of disease activity at the time of the decision to reduce the dose were on average DAS28 2.31±0.52, whereas at the moment the data were collected it was 2.32±0.72 (no significant difference). As expected when comparing the mean DAS28 between the low dose group and standard dose group, significantly lower values were observed in the low dose group compared to the standard system. Significant differences were also seen in the mean CRP and ESR values in both groups. Similarly, the percentage of patients in remission or low disease activity was significantly higher in the low dose group (Table 4). In RA patients, the reason for dose reduction was disease remission (DAS28≤2.6) in 32 patients (91.4%) and low activity (DAS28 3.2 and >2.6) in 3 (8.6%). The mean time of dose reduction in these patients was 13.6±11.9 months.
Characteristics of Patients With Rheumatoid Arthritis With Biologic Therapy at Standard or Reduced Dose.
| Total patients, n=82 | Patients with biological standard dose, n=47 | Patients with reduced biological dose, n=35 | P | |
| Age (years) | 57.1±12.0 | 57.5±11.4 | 56.5±12.9 | NS |
| Gender (female), n (%) | 70 (85.3) | 43 (91.4) | 28 (80) | NS |
| Time since onset of disease (years) | 8.0±15.0 | 8.1±15.0 | 7.9±15.0 | NS |
| Presence of erosions, n (%) | 66 (80.4) | 40 (85.1) | 26 (74.3) | NS |
| Rheumatoid factor and/or anti-CCP, n (%) | 72 (87.8) | 41 (87.2) | 31 (88.6) | NS |
| Previous DMARD (n) | 1.0±2.7 | 1.0±2.8 | 0.9±2.6 | NS |
| Concomitant DMARD, n (%) | 55 (67) | 35 (74.4) | 20 (57.1) | <.0001 |
| Methotrexate | 46 (56) | 29 (61.7) | 17 (48.6) | |
| Leflunomide | 8 (9.7) | 5 (10.6) | 3 (8.6) | |
| Sulfasalazine | 1 (1.2) | 1 (2.1) | – | |
| Concomitant corticosteroids, n (%) | 38 (46.3) | 30 (63.8) | 8 (22.9) | <.0001 |
| Current biological therapy duration (m) | 48.2±33.9 | 46.9±37.0 | 50.1±29.6 | NS |
| Current biological drug, n (%) | ||||
| Anti-TNF, n (%) | 57 (69.5) | 29 (61.7) | 28 (80) | NS |
| Non anti TNF, n (%) | 25 (30.5) | 18 (38.3) | 7 (20) | |
| ESR (mm/h) | 17.3±14.8 | 22.7±17.1 | 10.1±5.8 | <.0001 |
| CRP (mg/dl) | 0.6±1.0 | 0.9±1.2 | 0.17±0.27 | <.0001 |
| DAS28 index | 2.8±1.0 | 3.2±1.0 | 2.28±0.66 | <.0001 |
| Remission (DAS28≤2.6), n (%) | 45 (47.3) | 16 (34) | 29 (82.9) | <.0001 |
| Low activity or remission (DAS28≤3.2), n (%) | 59 (72) | 28 (59.6) | 31 (88.6) | .001 |
Anti-CCP: cyclic citrullinated peptide antibody; DMARD: disease-modifying anti-rheumatic drug, m: months, n: number, NS: not significant, CRP: C-reactive protein, TNF: tumor necrosis factor, ESR, erythrocyte sedimentation rate.
In bold, statistically significant data.
In patients with reduced dose, the therapeutic decision at the time of data collection was: keep the dose reduced in 94.3% (66 patients), further reduce the dose of the drug in 3 patients (4.3%) and increase it in one patient (1.4%) according to the disease activity, as assessed by clinical judgment and normal disease indexes.
DiscussionIn this study we analyze the frequency and characteristics of patients treated in our hospital who were being treated with low doses of biologics in clinical practice conditions, having achieved adequate control of the disease, comparing them with those receiving standard doses. This transversal analysis confirms that a significant number of patients (45.7%) treated with biological drugs receive a reduced dose, and the said dose is applied to various inflammatory arthritis, and many such patients were receiving low doses for a relatively prolonged period of time (mean 17 months), maintaining remission or low disease activity. There are differences between patients with reduced doses compared to standard-doses regarding concomitant use of DMARDs, corticosteroids and NSAIDs. In the case of RA and, as expected, lower dose patients showed a clear reduced disease activity and high rates of remission compared with patients with standard doses.
A clinical situations facing today's clinical rheumatologist is how to decide to follow the therapeutic approach in patients with adequate control of the disease under biological therapy, remission or low activity extended in time. Although most patients with RA and other inflammatory arthropathies are not in remission under these biological agents,12 there is no doubt that this goal, or that of attaining a low disease activity, can be achieved in a significant number of cases.
Biological drug withdrawal in these cases is followed, in most cases, of a clinical relapse within a few months.6,7 However, observational studies of therapeutic strategy, such as the BEST study demonstrate that the biological removal is possible in a high percentage of cases; in this study, more than half of the patients who had taken an initial strategy of infliximab plus methotrexate left infliximab after reaching low disease activity.13 However, this possibility is more evident in those patients in whom the biological is administered as a first option,14 something unusual in the current strategy for RA treatment, where the biological agent is virtually always used when one or more synthetic DMARD9 have failed. However, such removal appears to be possible also in the case of RA in patients after failure to DMARDs, as suggested by a Japanese study,15 although cases which would benefit from the removal would be those with little time since onset of RA, but not those with a evolved disease as a new study by the Emery Group suggests.16
Under this prism of high probability of clinical recurrence in patients who leave biological therapy once remission is achieved, it sounds logical to take an intermediate position as would be the progressive dose reduction (or increase in the dosing interval) along with monitoring the disease activity. In the current EULAR recommendations for the management of patients with RA9 it is established that if a patient with RA achieves permanent remission (12 months?) and is able to suspend steroids, a strategy for biological dose reduction may be considered, especially if the patient is being treated with DMARDs. This recommendation, based on expert opinion, is being used empirically in clinical practice in different inflammatory arthropathies, with some groups contributing their own experiences, both in SA AR,8,17–20 or21,22 PsA with good results, although some results have been obtained only through conference papers. The SER, in their consensus document on the use of biological therapies in RA23 states that, in patients who have achieved remission, acceptable options are to maintain the biological agent, try to lower the dose, increase the dosing interval or even suspend the biological agent, as long as the patient remains in clinical remission for a time yet to be defined. However, in the consensus document for PsA it is stated that there is no current evidence to recommend biological reduction in this disease, although it can be considered individually.24 In the case of AS, the SER consensus postulates that in selected patients with minimal clinical activity such a reduction may be considered.25
The first description in the literature on the results of the reduction of the dose of biological agents in patients with arthritis dates back to 2002 when a Dutch group found that in a small group of patients with RA treated with adalimumab, 76% were able to reduce TNF antagonist doses, with monitoring of the disease activity with DAS28.26 The same group of authors stated in a recent editorial10 that dose reduction is possible in patients with RA, arguing that there is overtreatment in a significant number of patients in whom the dose may be reduced while achieving the same clinical benefit, and in this sense monitoring of drug levels could be useful to the clinician. These same authors also proved that in most patients with RA who had increased the dose of infliximab 3–5mg/kg, a position with questionable evidence,27 the dose can be reduced to 3mg/kg without increasing disease activity as measured by DAS28.28
To the best of our knowledge only one randomized trial existed earlier which analyzes whether reduced biological dose can maintain control of the disease in patients who have achieved a low disease activity with standard biological dose. The PRESERVE studied,29 RA patients with moderate activity as measured by DAS28, who had achieved adequate control of RA (DAS28<3.2) with standard doses of etanercept (50mg/week) plus methotrexate and who were randomized to three treatment arms: follow standard dose, mid-dose (25mg/week) and complete withdrawal and where then followed for one year. Most patients still on etanercept maintained the low activity and no differences between those receiving a standard dose or reduced dose (82.6% and 79.1% respectively) was seen, while in the patients who were withdrawn from the biological this percentage is significantly reduced (42.6%). Interestingly, there are also differences in radiographic progression between the two groups of patients treated with etanercept. In fact, a French retrospective study showed a similar clinical response in patients with RA and AS when comparing – etanercept 25mg 2 times/week – with etanercept 25mg/week.30 However, although the PRESERVE study is interesting and scientifically supports the strategy of etanercept dose reduction is possible in a significant number of patients with RA, it must be remembered that the patients included had moderately active RA, so these results cannot be extrapolated to patients who begin with high activity (DAS28>5.1), a circumstance which occurs for example in 62% of RA patients in our service (unpublished data) or those starting other biological drugs. It is unknown if there are similar studies in patients with PsA. As for the SA or axial spondyloarthritis, there is an ongoing national randomized study of dose reduction with TNF antagonists in 190 patients sponsored by the Spanish Foundation of Rheumatology and Spanish Society of Clinical Pharmacology (REDES study) that will undoubtedly provide data on this issue.31
The reduced biological dose was used in this series in a similar percentage in the 4 groups of patients classified according to diagnosis, though somewhat lower in patients with RA. A recent study in patients with early disease who had gone into remission with adalimumab at a standard dose (40mg/2 weeks), points out that the ability to maintain this remission with increased dosing intervals (adalimumab 40mg/4 weeks) would be much more frequent in PsA than in RA22; however, it should be noted that our strategy of dose reduction in these diseases never contemplated the move to a monthly dose without first undergoing adalimumab treatment at a dose of 40mg/3 weeks. Moreover, it is interesting that in our study there were no differences in the time of disease progression or duration of current biological therapy between the groups with and without dose reduction, although it was less frequent to see the use of prior biological agents in patients with dose reduction. It is of interest, in the case of RA, to see the reduced use of concomitant DMARDs in patients with reduced doses, a fact which certifies that patients with biological agents alone could also benefit from the reduction of dose. As expected, due to the decision to reduce the dose when the patient was in permanent clinical remission and occasionally in low activity, reduced dose RA patients showed high remission rates, lower disease activity and lower frequency of concomitant treatment with corticosteroids than patients with standard doses, all data that would support the fact that such a reduction is possible in these patients to maintain adequate control of the disease.
The present study, however, has a number of limitations that should be mentioned. First, it is an observational study of clinical practice, with a relatively small number of patients without any pre-established protocol, so the decision and the moment to proceed with dose reduction are at the discretion of the investigator only. Secondly, the dose reducing regimen was also empirical and was done progressively, while always taking into account the activity of the disease and, in the case of clinical relapse, returning to the standard dose and, in fact, a significant percentage of patients in this series had previously tried to reduce the dose, but were back with the standard dose after the target had not been achieved; we did not specifically examine predictors of recurrence in these patients. Third, it is a cross-sectional study, so we do not know whether this reduction may or may not be sustained over time or result in a loss of clinical benefit in the medium to long term, not only in terms of disease activity, but also in functional capacity, structural damage or even on the immunogenicity of these drugs. However, the fact that the average dose reduction in these patients was relatively prolonged (17 months) at the time of the evaluation and the fact that these patients remained in remission or low activity strongly suggests that such a reduction is possible in a number of patients followed in our clinical practice. Fourth, our experience with reduced biological dose is based primarily on etanercept, adalimumab and tocilizumab and to a lesser extent, infliximab. We have no experience in terms of dose reduction, for obvious reasons, with other biological drugs such as golimumab or certolizumab, nor with non-anti-TNF agents such as abatacept and rituximab. In the latter case, randomized trials with DMARD failing patients suggest a similar clinical efficacy between – a dose of 1g (2×500mg) and 2g (2×1000mg).32 In the recent EULAR 2012 meeting held in Berlin, 2 studies examined rituximab dose reduction to 500mg from the second cycle with conflicting results.33,34
Finally, we evaluated the incidence of adverse effects or costs associated with dose reduction. Although it is unknown if dose reduction would achieve a significant reduction of adverse effects, it is clear that it reduces costs, at least in terms of direct costs and could be cost-effective.35 In fact, we believe this strategy has allowed to greatly control expenditure on biologicals in our hospital.
In summary, it is possible to reduce the dose of biological agents in patients with inflammatory arthritis in remission or low activity initially treated with standard doses, achieving adequate control of the disease in many cases of routine clinical practice. However, there are virtually no studies designed to determine the effectiveness of dose reduction strategies, so this practice is still empirical for clinical rheumatologist. The publication of some studies, and the strategies on dose reduction protocols are needed to support this clinical practice that is commonly used in many rheumatology services and, possibly, will be increased in the context of the current economic crisis and strict control of health spending. It seems natural that, in all the optimization strategies of the different drug treatment for inflammatory arthritis, reducing the dose of biological agents should play a predominant role.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that experiments have not been performed on humans or animals.
Data confidentialityThe authors state that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflict of interest.
José Inciarte-Mundo and M. Victoria Hernández have participated equally in the design and elaboration of the study and manuscript.
Please cite this article as: Inciarte-Mundo J, Hernández MV, Rosario V, Ruiz-Esquide V, Cabrera-Villalba S, Ramírez J, et al. Reducción de dosis de terapias biológicas en enfermedades reumáticas: análisis descriptivo de 153 pacientes en condiciones de práctica clínica. Reumatol Clin. 2014;10:10–16.