Axial spondyloarthritis (axSpA) are musculoskeletal diseases with different manifestations. In clinical practice, variability, and limitations in the collection of the outcomes required for follow-up have been observed. The objective of the CREA project was to agree on improvement strategies for the initial assessment and follow-up of patients with axSpA in Spain.
Materials and methodsA survey with 33 questions was conducted by a representative sample of rheumatologists on clinical practice, resources, and present limitations in the follow-up of patients with axSpA. The results of the survey were discussed in 10 regional meetings, and 105 strategies were proposed and evaluated through a Delphi consensus in which 85 experts participated.
ResultsThe lack of time for clinical visits, the lack of nurses and/or support staff and the delay in performing the imaging tests were the most prominent limitations in the follow-up of patients with axSpA. One hundred and five strategies were proposed related to the evaluation of disease activity, physical function, quality of life and disease impact, to the evaluation of comorbidities and extra-articular manifestations, laboratory tests; imaging tests, physical examination and metrology. Of the total, 85 were considered highly advisable. No regional differences were found.
ConclusionsThe proposals agreed upon as highly advisable in the present study are applicable to the entire national territory, allow tighter and more homogeneous monitoring of the patients with axSpA, facilitate more comprehensive management of the disease, and respond to the unmet needs detected in the initial survey.
La espondiloartritis axial (EspAax) es una enfermedad musculoesquelética con manifestaciones diversas. En la práctica clínica se ha observado variabilidad y limitaciones en la recogida de las variables necesarias para su seguimiento. El objetivo del proyecto CREA fue consensuar estrategias de mejora para la valoración inicial y el seguimiento de los pacientes con EspAax en España.
Materiales y métodosSe realizó una encuesta con 33 preguntas a una muestra representativa de reumatólogos expertos del territorio español sobre la práctica clínica, los recursos y las limitaciones actuales en el seguimiento de los pacientes con EspAax. En 10 reuniones regionales se discutieron los resultados de la encuesta y se propusieron 107 estrategias que fueron valoradas mediante un consenso Delphi en el que participaron 85 expertos.
ResultadosLa falta de tiempo en consulta, de personal de enfermería y/o de apoyo, y el retraso en la realización de pruebas de imagen fueron las limitaciones más destacadas en el seguimiento de los pacientes con EspAax. Se propusieron 202 estrategias relacionadas con la evaluación de los índices de calidad de vida e impacto de la enfermedad; las comorbilidades y manifestaciones extraarticulares; las pruebas de laboratorio; las pruebas de imagen; la exploración física y metrología; y los índices de actividad y función. De todas, 54 se consideraron altamente aconsejables. No se encontraron diferencias regionales en los valores de consenso.
ConclusionesLas propuestas consensuadas como altamente aconsejables en el estudio actual son aplicables a todo el territorio nacional, permiten realizar un seguimiento y control más estrecho y homogéneo de los pacientes con EspAax, facilitar un manejo integral y responden a las necesidades no cubiertas detectadas en la encuesta inicial.
Axial spondyloarthritis (axSpA) is a musculoskeletal disease that can have different clinical manifestations. Its main symptom is chronic back pain, usually inflammatory, accompanied by stiffness, which improves with physical exercise1. axSpA mainly affects the axial skeleton, and its most characteristic radiological lesion is sacroiliitis2,3. Patients with axSpA may also present with extra-articular manifestations, both musculoskeletal (arthritis, enthesitis and dactylitis) and non-musculoskeletal (uveitis, psoriasis and inflammatory bowel disease)4,5. The axSpA concept encompasses patients with ankylosing spondylitis (AS) and patients with non-radiographic xxSpA (nr-axSpA)6, who do not have radiographic sacroiliitis according to the modified New York criteria for AS but are classified by virtue of the presence of inflammatory lesions on magnetic resonance imaging (MRI) of sacroiliac (SI) joints or the presence of human leukocyte antigen B27 (HLA-B27) along with other typical features of spondyloarthritis7. AxSpA has a significant impact on quality of life (QoL)8 and is associated with various comorbidities9.
The worldwide prevalence of axSpA, including radiographic and non-radiographic patients, ranges between 20 and 161 per 10,000 people10. The prevalence of AS is between 9 and 30 per 10,000 people3. In Spain, the prevalence of AS has been estimated at 26 per 10,000 people11.
In routine clinical practice, a wide variability and insufficient collection of the variables necessary for the follow-up of patients with axSpA has been reported12,13. At the national level, it has been observed that 87% of clinical records do not include a disease activity index or a global assessment of the patient; 84% do not include a functional index; and 60% do not contain an assessment of possible joint involvement12. With the aim of standardising clinical practice and improving the management and prognosis of axSpA, guidelines for patient follow-up have been published both nationally and internationally1,14,15. In Spain, moreover, an action chart has been developed to facilitate the assessment of patients with axSpA16.
The objective of the CREA project was, first, to understand the current clinical practice, available resources and limitations in the follow-up of patients with axSpA and, in a second phase, to develop and agree, through a representative sample of expert rheumatologists, on strategies to improve the follow-up of patients with axSpA in Spain.
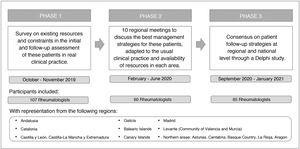
MethodsThe CREA project is a qualitative and quantitative study developed over 3 distinct phases: a nationwide survey, a series of regional meetings to discuss the survey results and propose actions, and a Delphi consensus to validate the proposed strategies, understood as a series of actions to be carried out to improve the management of patients with axSpA. The strategies refer to the set of assessments, tests and evaluations of the disease that it would be desirable to implement in clinical practice to improve the management of patients with axSpA (Fig. 1).
Resources and constraints surveyDuring October and November 2019, a nationwide survey was conducted on the specialised care of patients with axSpA, with the aim of finding out the existing resources and limitations in routine clinical practice. The survey was designed by a scientific committee composed of 10 experts and was divided into 4 sections: (a) Participating physician profile, (b) Patient management in the practice, (c) Patient assessment, and (d) Limitations encountered in clinical practice. The survey comprised 33 questions including different items. 107 rheumatologists with demonstrated interest and experience in the management of the pathology, members of the Spondyloarthritis Study Group of the Spanish Society of Rheumatology (GRESSER), invited by the scientific committee and representing the different regional areas, participated in the survey (Table 1).
Distribution of regional participation in the different phases of the CREA project.
| Sample total | Andalusia | Catalonia | Castilla y Léon, Castilla-La Mancha and Extremadura | Galicia | Balearic islands | Canary islands | Madrid | Levantea | Northern areab | |
|---|---|---|---|---|---|---|---|---|---|---|
| Participation by phases | ||||||||||
| Phase 1: Resources and constraints surveys, n (%) | 107 (100%) | 12 (11.4%) | 12 (11.4%) | 7 (6.7%) | 14 (13.3%) | 4 (3.8%) | 9 (8.6%) | 12 (11.4%) | 21 (20.0%) | 16 (15.2%) |
| Phase 2: Regional meetings, n (%) | 60 (100%) | 5 (8.3%) | 6 (10.0%) | 6 (10.0%) | 6 (10.0%) | 5 (8.3%) | 5 (8.3%) | 8 (13.3%) | 13 (21.6%) | 6 (10.0%) |
| Phase 3: Management strategies consensus, n (%) | 85 (100%) | 9 (10.6%) | 13 (21.7%) | 6 (10.0%) | 9 (15.0%) | 1 (1.7%) | 8 (13.3%) | 10 (16.7%) | 19 (31.7%) | 10 (16.7%) |
The characteristics of the participants and the results obtained were described using frequency tables for nominal variables and measures of central tendency and dispersion for continuous variables. Data were analysed globally and by region.
Regional meetingsBased on the results of the survey, between February and June 2020, 10 regional meetings were held to propose and discuss strategies, and adapt them to routine clinical practice and the availability of resources in each area. Sixty of the rheumatologists (approximately 6 experts per meeting), who had previously participated in the survey, were involved. A total of 105 patient follow-up strategies were defined (11 general and 94 specific to patients with axSpA) and, with the advice of the scientific committee, a Delphi questionnaire was developed with 6 sections: (a) Quality of life and disease impact indices, (b) Assessment of comorbidities and extra-articular manifestations, (c) Laboratory tests, (d) Imaging tests, (e) Physical examination and metrology, and (f) Activity and function indices (Appendix B Supplementary material 1).
Delphi consensusTwo waves of questionnaire assessment were conducted between September 2020 and January 2021; 92 rheumatologists participated in the first wave and 85 in the second wave, all of whom had previously participated in the resources and constraints survey.
For interpretative purposes, the proposed strategies were categorised as “highly desirable”, “desirable” or “dispensable”, and the level of agreement and desirability were assessed using a Likert-type scale. Ratings from 1 to 3 were considered as “disagree”, 4 to 6 as “neutral” and 7 to 9 as “agree”. Consensus was defined as 70% or more of the participants agreeing with the same answer.
ResultsParticipants profileThe mean age of the resources and constraints survey was 48.3 years with a mean of 20 years of experience in their specialty. Fifty-five per cent were women. Ninety-four per cent worked in a public health hospital.
The mean of patients visited per year was 1,517, 21% of whom were patients with axSpA (322 patients visited per year) (Table 2). Sixty per cent of these had been referred from primary care and 21% from traumatology and rehabilitation.
National survey participant profile.
| Participant description | N = (107) |
|---|---|
| Age, mean (min., max.) | 48.3 (30. 67) |
| Type of centre | |
| Public hospital | 94.0% |
| Private hospital | 3.0% |
| Outpatient specialty centre | 3.0% |
| Years of experience, mean (min., max.) | 20 (5. 40) |
| Patients attended in one year, mean (min., max.) | 1517.4 (400, 5000) |
| Patients with axSpA attended in one year, n (% of total patients) | 322 (21.0%) |
| Available resources | |
| axSpA | |
| Initial visit | |
| Time available at consultation, % | 21–30 min, 48.6% |
| Optimum time, mean (min., max.) | 33,0 (15. 60) |
| Follow-up visit | |
| Time available at consultation, % | 10–15 min, 66.4% |
| Optimum time, media (min., max.) | 20.5 (10. 30) |
| Rheumatology residence, Yes % | 54.0% |
| Monographic axSpA consultation, Yes % | 30.8% |
| Type of monographic consultations | |
| Spondyloarthritis | 76.0% |
| Rheuma-ophthalmology | 58.0% |
| Availability of rheumatology nurse, Yes % | 77.6% |
| Tasks performed by rheumatology nurse | |
| Treatment education | 95.2% |
| Treatment analytical controls | 62.7% |
| Measurement of cardiovascular risk factors (weight, BMI, abdominal perimeter,BP) | 61.4% |
| Performing of axSpA, peripheral ASpA and PI | 51.8% |
| Examination of joints and spinal metrology | 37.3% |
| Formation of patients into group chats | 24.1% |
| Computerised clinical record, yes % | 99.1% |
| Assessment of axSpA patients in a digital database, yes % | 46.7% |
Fifty-four per cent of the participants had rheumatology residents, although differences were observed between the areas (from 78% in the Canary Islands to 0% in the Balearic Islands). Thirty-one per cent had a monographic axSpA consultation and 78% had rheumatology nurses, who in 95% of the cases were involved in education about treatments. Ninety-nine per cent had computerised medical records in their centres and 47% recorded the assessment of axSpA patients on a digital database (Table 2).
Patient management in the practiceForty-nine per cent of the participants specified that the time available for first visits for patients with axSpA is 21–30 min and considered that the mean optimal time for such a visit should be 33 min. Sixty-six percent of them stated that the time available for follow-up visits is 10–15 min and the mean optimal time should be 20.5 min.
Forty-nine per cent of the participants agreed that axSpA active patients are assessed every 3 months, and 66% assess patients with axSpA inactive or minimally active every 6 months.
Patient assessmentPhysical examination and metrology is mainly performed by the physician. Examination of the hips, occiput/wall distance and cervical rotation are performed by more than 90% of participants. Only 66% reported performing the swollen and painful joint count and 51% of participants reported performing all the proposed physical examination and metrology tests.
Sixty-eight per cent, 62% and 43% stated they did not test abdominal circumference, fibromyalgia points and blood pressure, respectively. Weight, height and body mass index (BMI) assessment was not performed in 24% of cases and was performed in 60% of cases by the nursing staff.
The visual analogue pain scale (VAS) was the most commonly used pain scale in the assessment of patients according to 93% of the participants. About 75%–80% assess activity using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (75%) and Ankylosing Spondylitis Disease Activity Score (ASDAS) (74%). Function is assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI) (82%). The physician is usually responsible for the assessment of activity and function indices.
More than 90% reported using lateral spine radiography, plain pelvis radiography, MRI of the spine and/or MRI of the sacroiliac joints in the assessment of the patient with axSpA. Plain pelvis X-ray and sacroiliac joint MRI are mostly performed at diagnosis, spine X-ray at diagnosis or periodically every 2 years, and spine MRI occasionally, depending on the patient’s clinical presentation.
Almost all the proposed comorbidities and extra-articular manifestations were assessed by more than 70% of the participants. Ninety-nine per cent and 97% of respondents reported assessing, respectively, predictors of structural damage, and response factors to biologic therapy.
The most commonly used index for the impact of the disease was the Patient Global Assessment of the disease (PtGA), with 46% of the experts agreeing. Forty-two percent of them did this every 3 months and 44% every 6 months. Forty-two per cent of them did so every 3 months and 44% every 6 months.
Constraints in clinical practiceThe most prominent limitations according to the experts regarding the management and assessment of patients with axSpA were the lack of time in the clinic, the lack of nursing and/or support staff, and the delay in performing imaging tests (Table 3).
Limitations reported by experts regarding the management and assessment of patients with SpAax. National resources and constraints survey.
| N = 107 | |
|---|---|
| Lack of consultation time | 3.79 |
| Lack of nursing and/or support staff | 3.66 |
| Delay in performing imaging tests | 3.49 |
| Lack of access to imaging tests | 1.44 |
| Lack of knowledge of indices/questionnaires for patient assessment | 1.5 |
| Lack of adequate digital resources for data collection | 3.01 |
| Lack of communication with MAP | 2.9 |
| Lack of multidisciplinary communication with other specialties involved in these patient profiles | 2.48 |
| Others | 2.6 |
A total of 94 specific strategies were defined for the follow-up of axSpA patients and 11 for SpA (spondyloarthritis) patients, of which 77 (82%) and 10 (91%) were agreed, respectively (Table 4). There were no significant differences in the level of consensus obtained in the different regional areas (Appendix B Supplementary material 2). A total of 54 of the proposed strategies for axSpA patient follow-up were considered highly desirable (Table 5).
Consensus reached in each wave of the Delphi study.
| Follow-up strategies of the patient with axSpA | ||||||
|---|---|---|---|---|---|---|
| Wave 1 | Wave 2 | |||||
| Totala | General | axSpA | General | axSpA | ||
| Total strategies | 202 (100%) | 11 (100%) | 94 (100%) | 202 (100%) | 11 (100%) | 94 (100%) |
| Consensus reached (≥70% in agreement) | 154 (76%) | 10 (91%) | 68 (72%) | 171 (85%) | 10 (91%) | 77 (82%) |
| Agreement consensus | 154 (76%) | 10 | 68 (72%) | 171 (85%) | 10 | 75 (80%) |
| Disagreement consensus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%%) |
| Consensus not reached (<70% agreement) | 48 (24%)b | 1 (9%) | 26 (28%) | 31 (15%) | 1 (9%) | 19 (20%) |
Highly recommended strategies for the follow-up of patients with axSpA. Results of the Delphi consensus.
| Frequency | Highly desirable strategies | Disagree (1-2-3) (%) | Neutral (4-5-6) (%) | Agree (7-8-9) (%) |
|---|---|---|---|---|
| Quality of Life Indices/Disease Impact (PRO) | ||||
| Assess at least one quality of life index, at least once per yeara | 1.10 | 4.30 | 94.60 | |
| Patient Global Assessment (PtGA) at each visit | 4.70 | 14.10 | 81.20 | |
| Physical examination and metrology | ||||
| Important to be aware of the possibility of peripheral involvement in patients with axSpA | .00 | .00 | 100.00 | |
| Intermalleaolar distance | 3.50 | 4.70 | 91.80 | |
| At each visit | Hip examination | 1.20 | 1.20 | 97.60 |
| Ask about history of red eye | 3.50 | 5.90 | 90.60 | |
| Take into account the skin through anamnesis | 5.90 | 9.40 | 84.70 | |
| Perform a count of symptomatic enthesitis | 3.50 | 11.80 | 84.70 | |
| Perform count of swollen/painful joints that are symptomatic | 4.70 | 10.60 | 84.70 | |
| Perform specific dactylitis count and record it separately from the joint count | 5.90 | 16.50 | 77.60 | |
| Once a year | Assess cervical mobility by performing at least a tragus-wall or occiput-wall | 0.00 | 3.50 | 96.50 |
| Cervical rotation | 1.20 | 4.70 | 94.10 | |
| Modified Schöber test | .00 | 5.90 | 94.10 | |
| Thoracic extension | 4.70 | 2.40 | 92.90 | |
| Finger-floor distance | 3.50 | 3.50 | 92.90 | |
| Ask about bowel symptoms. If no symptomatology. on an annual basis | 9.40 | 14.10 | 76.50 | |
| Assessment of comorbidities and extra-articular manifestations | ||||
| Assess cardiovascular risk factors, regardless of whether the assessment is done by the rheumatology service, nursing or is done by primary carea | .00 | 1,20 | 98.80 | |
| Have nursing staff assess cardiovascular risk factors (weight, height, BMI, blood pressure)a | .00 | 5.90 | 94.10 | |
| Review the patient’s medical history at each visit, to assess comorbidities, even if they are not asked directly to the patient at the consultationa | 1.20 | 8.20 | 90.60 | |
| At each visit through anamnesis | Cardiovascular disease | 3.50 | 10.60 | 85.90 |
| Kidney failure | 2.40 | 11.80 | 85.90 | |
| Obesity/overweight | 2.40 | 12.90 | 84.70 | |
| Uveitis (confirmed by an ophthalmologist) | 3.50 | 11.80 | 84.70 | |
| High blood pressure | 2.40 | 14.10 | 83.50 | |
| Diabetes mellitus | 2.40 | 14.10 | 83.50 | |
| Tobacco | 4.70 | 12.90 | 82.40 | |
| Dyslipidaemia | 2.40 | 15.30 | 82.40 | |
| Inflammatory bowel disease (UC and Crohn’s) | 2.40 | 16.50 | 81.20 | |
| Metabolic syndrome | 2.40 | 16.50 | 81.20 | |
| Sedentary lifestyle. amount and type of exercise | 3.50 | 18.80 | 77.60 | |
| Alcohol | 4.70 | 18.80 | 76.50 | |
| Gastric ulcer | 11.80 | 14.10 | 74.10 | |
| Osteoporosis. if risk factors are present | 9.40 | 21.20 | 69.40 | |
| Once a year | Blood pressure | .00 | 4.70 | 95.30 |
| Ask the patient if he/she has psoriasis at baseline and annually thereafter | 1.20 | 3.50 | 95.30 | |
| Weight/height/BMI | 1.20 | 4.70 | 94.10 | |
| Activity and function indices | ||||
| Promote the role of rheumatology nurses to increase the use of activity and functiona | .00 | 2.40 | 97.60 | |
| At each visit | VAS pain (0–1) | 1.20 | 7.10 | 91.80 |
| Overall patient VAS | 2.40 | 8.20 | 89.40 | |
| Overall physician VAS (0–1) | 1.20 | 10.60 | 88.20 | |
| ASDAS and/or BASDAI: at least one of the two, at each visit | 2.40 | 10.60 | 87.10 | |
| ASDAS | 1.20 | 11.80 | 87.10 | |
| Minimum once a year if peripheral involvement | BASFI, minimum once a year | 4.70 | 12.90 | 82.40 |
| If peripheral involvement, DAPSA at every visit | 4.70 | 17.60 | 77.60 | |
| Lab tests | ||||
| At each visit | Reactive C protein, at each visit | .00 | 3.50 | 96.50 |
| Haemogram, at each visit | .00 | 3.50 | 96.50 | |
| General biochemistry with hepatic profile, at each visit | 1.20 | 2.40 | 96.50 | |
| Creatinine clearance/filtrate (MMDR-4), at each visit | 1.20 | 7.10 | 91.80 | |
| Erythrocyte sedimentation rate (ESR), every visit | 5.90 | 14.10 | 80.00 | |
| Every 6–12 months | Lipid profile, every 6–12 months, depending on risk profile | 000 | 1.20 | 98.80 |
| Once a year | Urine S. (systematic urinalysis), at least once a year | 3.50 | 8.20 | 88.20 |
| Imaging tests | ||||
| Peripheral X-rays of hands and feet if clinically present | 0.00 | 3.50 | 96.50 | |
| X-ray of the lateral cervical and/or dorsal and/or lumbar spine, at a frequency depending on progression factors, at least every 2–3 years | 1.20 | 3.50 | 95.30 | |
| Plain X-ray of the pelvis, at a frequency depending on progression factors, at least every 2–3 years | 2.40 | 7.10 | 90.60 |
Ninety-four point six per cent of the participants agreed that assessing at least one quality of life index once a year is highly advisable. Eighty-one point two per cent considered the global assessment of the patient using the PtGA at each visit.
All participants considered it highly advisable to assess peripheral involvement.
A hip examination, symptomatic enthesitis count, swollen/painful joint count, dactylitis count with a separate recording of the joint count were considered highly advisable at each visit.
It was agreed that annual assessment of cervical mobility, examination of cervical rotation, modified Schöber test, thoracic expansion and toe-floor distance was highly recommended.
Assessment of cardiovascular risk factors and the suitability of the nursing staff for this role were also considered highly advisable, as well as assessment of all extra-articular manifestations and comorbidities at each visit. Weight, height, BMI, blood pressure and occurrence of psoriasis could be assessed annually.
Global patient and pain assessment by VAS and ASDAS were recommended at each visit, while BASFI assessment was recommended annually.
Assessment of laboratory results at each visit, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), haemogram, general biochemistry with creatinine clearance or glomerular filtration rate, was considered highly advisable.
Cervical and/or dorsal and/or lumbar lateral spine and pelvis X-rays should be performed every 2–3 years, depending on progression factors. Peripheral X-rays of the hands and feet were considered necessary in case of clinical presence in the patient.
DiscussionThis paper proposes a total of 54 highly desirable strategies for optimal management in the initial assessment and follow-up of patients with axSpA, designed based on the results of a survey of current resources and limitations in the management of these patients. Although the strategies were presented at different regional meetings, no significant differences were found in the degree of consensus by region. Consequently, this study provides strategies for the proper follow-up and management of axSpA patients that are applicable nationwide.
Available resources are a key factor in the optimal follow-up of axSpA patients and can be a limiting factor. The national survey in the first phase of the present study showed a disparity between the desirable time for visits and the time currently spent by experts.
AxSpA is a potentially serious disease with very diverse manifestations, which generally requires multidisciplinary management1. In our study, insufficient human resources were found to be available: almost half of the participants had no residents and one in four had no specialised nursing staff. On the other hand, there seems to be a certain limitation in the tasks performed by the nursing staff, as they are scarcely involved in physical and metrological examinations, nor in the assessment of activity and function indices. In this respect, the promotion of specialist rheumatology nurses was considered a highly advisable strategy to increase the use of activity and function indices.
AxSpA poses a high health, economic and social burden that must be considered by the rheumatologist in the management of the disease. AxSpA has a major impact on the functional capacity, quality of life and productive capacity of some patients, even in early stages of the disease17,18. Consequently, follow-up of axSpA patients should include assessment of not only clinical findings, laboratory and imaging tests, but also quality of life and health through patient reported outcomes (PROs)1. While generic tools can be used, specific questionnaires such as the Ankylosing Spondylitis Quality of Life (ASQoL) or the Assessment of Spondyloarthritis International Society Health Index (ASAS HI)8,19,20, have been developed. More than half of the participants in the national survey reported not using any of the proposed health indices, and only one in 10 reported using ASAS HI for the assessment of their patients. However, it was considered highly advisable to assess patients’ health using an index at least once a year and to use the PtGA at each visit.
The heterogeneous presentation of axSpA and the coexistence of different clinical manifestations highlight the need to use composite assessment indices to obtain an overview of the disease21. Indices such as BASDAI and especially ASDAS are recommended to assess remission or minimal disease activity22. However, the limited use of indices and PROs has been previously described in the literature12,13,23. Since one out of 4 participants reported not using them in the national survey, the Delphi study considered it highly recommended to use at least one of the two disease activity assessment indices at each follow-up visit. Apart from the composite indices, the physician’s global assessment is crucial in the evaluation of the patient’s condition22. The physician’s global assessment at each visit was considered highly desirable, as well as the patient’s global assessment and pain assessment, all using VAS. The annual use of the BASFI questionnaire as a measure of patients’ functional capacity17 was also considered highly advisable.
Approximately 40% of axSpA patients experience one or more extra-articular manifestations during the course of the disease5. In addition, most of these patients also have at least one comorbidity9,24. The prevalence of comorbidities is higher in axSpA patients than in controls, and in the specific case of heart failure or depression may be up to 80%9. These comorbidities have an impact on function, quality of life and work productivity and even on patient survival25–27, and may condition therapeutic decisions. Therefore, it was considered highly advisable to assess comorbidities, cardiovascular risk and extra-articular manifestations of sxSpA.
Since radiographic sacroiliitis may be a late finding in the course of axSpA, MRI can help to detect signs of inflammation before radiographic structural damage appears and to assess patients for early therapeutic interventions1,4,28. Although the use of MRI can provide additional information to the clinical and analytical evaluation of axSpA, its use is not routinely indicated in the monitoring of disease activity22,29. Therefore, the imaging tests considered highly advisable for follow-up in the Delphi consensus were plain pelvis and lateral spine radiography, in line with current recommendations.
This study has certain limitations. Firstly, the sample of participants in the Delphi consensus may not be representative of the national rheumatology community. Although experts were selected from the different regions of the territory, the representation of each region was not homogeneous, which may limit the ability to detect differences between regions. Secondly, the implementation of some of the highly recommended strategies may not be feasible due to the limited resources available in some centres.
ConclusionsThe proposals agreed as highly desirable in the current study respond to the unmet needs detected in the national survey and discussed in the regional meetings held. These proposals are aimed at closer and more homogeneous monitoring and control of patients with axSpA, as well as facilitating comprehensive patient care. As no differences were found in the level of consensus at regional level, they would be applicable to the entire national territory.
FundingNovartis Farmacéutica S.A. has financially supported the project development, medical writing assistance and journal processing fees for this article. The sponsor had no role in the design of the study, the analysis or interpretation of the data, the writing of the article or the decision to submit the article for publication.
Conflict of interestsThe authors have on conflict of interests to declare related to this study.
To all the expert rheumatologists who participated in the regional meetings: Adela María Gallego Flores, Agustí Sellas i Fernández, Agustín Javier Alegre López, Alfonso Fernando Corrales Martínez, Ana Lafont Ruzafa, Ana Pérez Gómez, Ana Urruticoechea Arana, Àngels Martinez Ferrer, Antonio Álvarez de Cienfuegos Rodríguez, Antonio Juan Mas, Arantxa Conesa Mateos, Azucena Hérnandez Sanz, Beatriz González Álvarez, Carlos Alberto Montilla Morales, Carlos García Porrúa, Carlos Javier Rodríguez Lozano, Carlos Manuel González Fernández, Cristina Campos Fernández, Cruz Fernández, Delia Reina Sanz, Elisa María Trujillo Martín, Emma Beltrán Catalán, Erardo Ernesto Meriño Ibarra, Francisco José Maceiras Pan, Gloria Candelas Rodríguez, Jaime Calvo Alën, Javier Calvo Catalá, Jesús Rodríguez Moreno, José Ángel Hernández Beriain, José Antonio Mosquera Martínez, José García Torón, José Miguel Senabre Gallego, José Ramón Maneiro Fernández, Juan Moreno Morales, Laura González Hombrado, Luis Espadaler Poch, Luis Fernández Domínguez, Luis Francisco Linares Ferrando, Manuel José Moreno Ramos, María Concepción Castillo Gallego, María Cristina Lerin Lozano, María José Moreno Martínez, María Luz García Vivar, María Paz Martínez Vidal, Marta Valero Expósito, Meritxell Fernández Matilla, Mireia Moreno Martínez-Losa, Olga Sánchez González, Raquel Hernández Sánchez, Ricardo Gutiérrez Polo, Sara Manrique Arija, Sergio Antonio Rodríguez Montero, Sergio Ramon Machin García, Teresa Font Gayá, Tomás Ramón Vázquez Rodríguez, Vicente Aldasoro Cáceres, Yolanda Cabello Fernández, Julio Antonio Medina Luezas, Isabel de la Morena Barrio and José Campos Esteban.