The efficacy shown by biological therapy with tumor necrosis factor (TNF) antagonists has led in the recent years to its increased and extended use in different inflammatory arthropathies. Initially, safety studies of these drugs were mainly focused on the risk of infection and the development of malignancies. Recently, several cases of skin lesions induced by anti-TNF drugs have been reported with an increased incidence, highlighting the importance of the skin as a major target of the side effects of these drugs. In addition to skin lesions directly related to drug administration there is a wide spectrum of skin lesions of different morphology and etiology, especially the development of cutaneous immune-mediated conditions, an emergent phenomenon associated with this treatment. We describe the main skin lesions associated with treatment with anti-TNF drugs according to an extensive review of the literature.
La eficacia mostrada por los fármacos antagonistas del factor de necrosis tumoral (TNF) en los últimos años ha condicionado un aumento de su utilización en diferentes enfermedades inflamatorias articulares. Inicialmente, los estudios de seguridad de estos fármacos se focalizaron principalmente en el riesgo de infecciones y el desarrollo de neoplasias. Sin embargo, recientemente, se han comunicado diversos casos de lesiones cutáneas inducidas por estos fármacos, cuya incidencia ha ido en aumento, resaltando la importancia de la piel como uno de los principales órganos diana de los efectos secundarios de estos tratamientos. Además de las lesiones cutáneas relacionadas directamente con la administración del fármaco, se han observado un amplio espectro de lesiones cutáneas, de morfología y etiología diversa, llamando la atención el desarrollo de enfermedades cutáneas inmunomediadas como un fenómeno emergente relacionado con este tratamiento. Seguidamente se describen las principales lesiones cutáneas asociadas al tratamiento con anti-TNF y se realiza una revisión de la literatura.
During the last decade the development of so-called biological drugs has led to new therapeutic options in patients with multiple chronic inflammatory joint disease that result refractory to various treatments. The first and best-known is directed against the tumor necrosis factor (TNF), a proinflammatory cytokine implicated in the pathophysiology of various inflammatory diseases. The first anti-TNF drug available in our country was infliximab, a chimeric monoclonal antibody. Etanercept, which was subsequently approved, is a fusion protein of the TNF receptor, and adalimumab, a fully human monoclonal antibody. Recently, 2 new anti-TNF drugs have been approved: certolizumab pegol and golimumab. Although its initial indication was in patients with rheumatoid arthritis (RA),1–3 these drugs have proven effective in other inflammatory arthropathies such as ankylosing spondylitis (AS) or psoriatic arthritis (PsA), expanding its therapeutic indications, and even used “off-label” in other autoimmune pathologies due to its good results.4
Associated with the use of these drugs, the appearance of various adverse effects associated with their administration has also been described. Initially, the safety profile of anti-TNF was based on data from clinical studies used for approval, but then marketing data from notifications, alerts, clinical observational studies, registries or databases have contributed to better understand the safety of these drugs. In general, the biggest concern associated with anti-TNF therapy has been the development of infections, both common and opportunistic, but other side effects such as the development of malignancies, demyelinating disease, laboratory abnormalities and development of antibodies have also been the source of several studies and publications.5,6
The recent publication of several cases of “de novo” psoriasis induced after administration of anti-TNF warned about the importance of the skin as a potential target organ for adverse effects related to TNF7 antagonists. Subsequently, other articles have been published describing various skin disorders related to the administration of these drugs. Generally, most information comes from case reports or series of patients, but there is insufficient data from long-term studies or registries. The wide and growing use of these drugs in various chronic inflammatory diseases has shown not only an increased rate of dermal effects in clinical practice, but also a wide variability in the type of cutaneous adverse effect. Thus, there have been reports of psoriasis, eczematous eruptions, herpes zoster, bacterial and fungal infections, lichenoid eruptions, cutaneous vasculitis, alopecia, pemphigus, lupus erythematosus, vitiligo, and cutaneous lymphomas, among others.8–10 Below is a review of the main skin lesions related to the use of anti-TNF (Table 1).
Main Skin Lesions Associated to Treatment With TNF Antagonists.
| 1. Skin lesions related to the administration of treatment |
| - Erithema, urticaria, eczema, rash |
| 2. Skin infections |
| - Bacterial infections: cellulitis |
| - Viral infections: herpes zoster |
| 3. Skin neoplasia |
| - Non melanoma skin tumors: basocellular and spinocellular carcinoma |
| - Melanocytic tumors: benign melanocytic nevus |
| - Malignant melanoma |
| - Skin lymphomas: T cells, fungoid mycosis, Sezary syndrome |
| 4. Immune mediated diseases: |
| - «de novo» psoriasis and exacerbation of prior psoriasis |
| - Cutaneous lupus |
| - Alopecia areata |
| - Skin vasculitis |
| - Vitiligo |
| - Relapsing polychondritis |
| - Polymyositis/dermatomyositis |
| - Localized scleroderma (morphea) |
| - Granuloma anularis |
| - Lichen or lichenoid reaction |
| - Pemphigus |
The only skin lesions secondary to treatment with anti-TNF reported in clinical trials for drug approval are those directly related to drug administration, either intravenously as in the case of infliximab, or subcutaneously, and the rest of anti-TNF. Most of these reactions are mild to moderate without requiring, in most cases, the withdrawal of the drug. The type of associated skin reactions can be very diverse, the most common being: erythema, urticaria, eczema or rash, which may, in turn, be accompanied by pain or swelling. In many cases it could be drug hypersensitivity reactions that occur, with skin lesions as its main feature, which may imply a certain bias in their classification. In general, these lesions occur mainly in the first month of treatment and later descend in frequency. They have been reported with all anti-TNF drugs, although in different percentages. Thus, the appearance of rash has been described in 6.9% of patients receiving infliximab,1 reactions at the injection site have been documented in between 38%11 and 42%2 of patients receiving etanercept, and 15% of those with adalimumab.12 With new anti-TNF drugs, the incidence of skin reactions at the injection site is lower, 2.3% and 2.4% certolizumab pegol13 with golimumab,14 respectively. In general, this type of reaction is more frequently associated with subcutaneous administration drugs and is described more frequently with etanercept.
Skin InfectionsOther skin injuries frequently observed with the administration of anti-TNF are those associated with underlying infection. Thus, in a prospective study in patients with RA that analyzed the incidence and type of cutaneous adverse effects associated with anti-TNF, the most frequent were skin infections.10 Although at times it may present with systemic manifestations such as fever, in many cases the only apparent symptom may be a skin condition. The most frequently associated skin lesion is cellulitis, which can sometimes also affect soft tissue, and is usually of bacterial origin, and infection with herpes zoster, characterized by the appearance of painful blisters following the path of a nerve due to reactivation of previous infection by the varicella-zoster virus. Skin infections are also one of the most common infections seen in patients receiving anti-TNF therapy, as demonstrated in a retrospective study in 709 patients with chronic inflammatory joint diseases with anti-TNF treatment in which skin infections accounted for 21% of all infections, the second most common after the respiratory15 tract. Similarly, another study found a fourfold increase in the risk of skin and soft tissue infection in patients with RA treated with anti-TNF therapy compared to patients receiving DMARD.16 This is not surprising since TNF-α is a proinflammatory cytokine that plays an important role in innate immunity and protection against bacterial, viral and parasitic diseases, and its inhibition may increase the risk of infection, as described previously.17 Furthermore, studies in patients with RA treated with biological drugs show an increased risk of infection compared with those patients not receiving this type of therapy.18–20 But the high incidence of skin infections also suggest an important physiological role of TNF in host defense against pathogens at the level of the skin and soft tissues that is more relevant than in other areas.16 In addition to bacterial skin infections, recent studies have also shown an increased risk of viral infections in patients treated with biologic therapy, especially herpes zoster.21 It has also been postulated that this increased risk is more related to treatment with monoclonal antibodies such as infliximab and adalimumab than with etanercept.22 In any case we must take into account that RA patients have, in the end, an increased risk for developing herpes zoster in relation to the general population.23 In relation to the type of anti-TNF drugs related to the development of infection, infliximab has been shown in some studies to be associated with higher rates of skin infection than other drugs,15 although other studies show a slightly higher incidence related to adalimumab.22 However, when analyzing the overall risk of infections, some authors found no differences between the 3 anti-TNF blockers: etanercept, infliximab and adalimumab.16,24
Skin NeoplasmsAnother of the lesions observed in patients receiving anti-TNF therapy are skin tumors. The most common are non-melanoma skin tumors, mainly basal cell carcinoma and, less frequently, squamous cell carcinoma. When analyzing the overall risk of malignancy associated with anti-TNF therapy, some studies show an increase in its incidence,25 especially lymphomas,26 while others show an increase in its risk.27,28 Theoretically, inhibition of TNF-α may decrease the body's natural surveillance on the development of tumors, and prolonged its inhibition, as occurs with continued treatment with anti-TNF, that could be associated with worsening of the host control of premalignant lesions and increased neoplasia29 development. However, we must take into account that most studies analyze the risk of neoplasia in patients with RA, which fundamentally shows an increased risk of developing tumors, mainly lymphomas.26 In the case of skin tumors, the relationship between the administration of anti-TNF and the risk of skin carcinogenesis is still a subject that has not been clarified. Thus, different studies have shown non-melanoma skin neoplasms as the most frequent in these patients.28,30 Likewise, in another analysis of the incidence of cancer in patients with RA treated with biologics, there is a positive association between skin cancer and biological therapy.31 Similarly, in a long-term follow-up cohort of RA patients, there is an increased risk of nonmelanoma skin cancer in patients receiving anti-TNF.32 An increased risk of nonmelanoma skin neoplasia has also been shown in up to 70% in patients with RA treated with anti-TNF in relation to patients not receiving biologic therapy and an increased relative risk in relation to time of follow-up.27 Conversely, in an open study of 549 patients with RA treated with etanercept, followed for 5 years, we could not demonstrate an increased risk of cutaneous non-melanoma33 neoplasia. Neither did we find an increased risk of nonmelanoma skin neoplasia in a meta-analysis that examined the risk of anti-TNF therapy in patients with RA.24 Also, an analysis of British registry of biological drugs did not find an increased risk of malignancies associated with anti-TNF drugs,34 but did find an increased risk of basal cell carcinoma with an incidence of up to 10% in patients who previously had a basocellular34 carcinoma. Prior history of malignancy has been described as an increased risk factor for developing neoplasias.28,30 A recent meta-analysis of certolizumab and golimumab found no increased risk of cutaneous neoplasia with these drugs, although the results were short term, which is a major constraint to correctly interpret the results of this study.35
In addition to developing the most common skin cancers, basal and squamous cell carcinoma, cases of malignant melanoma after anti-TNF,36–38 and cases of benign melanocytic nevi benigno39 have also been reported,40 indicating a possible relationship between administration of anti-TNF and proliferation both benign and malignant melanocytic. However, the exact role of these drugs in tumorigenesis is not yet elucidated, as some authors have found an increased risk of melanoma up to 3 times higher in RA patients treated with methotrexate.41 However, it is recommended that patients receiving biologic therapy undergo identification of new pigmented lesions or changes in preexisting nevi to clarify their etiology and, if in doubt, consider its removal for histological analysis.
Other skin tumors that have been associated with anti-TNF treatment, though extremely rare, are cutaneous lymphomas. A few cases have been described in literature, generally T cell related, unlike most lymphomas related to biological therapy, which are of B cell lineage.42 They are presented as generalized skin lesions, red plaques or blue nodules.43,44 Among the various lymphomas, cases of mycosis fungoide45 and Sézary46 syndrome have also been reported. They generally have a better prognosis than other lymphomas, with reports of regression following withdrawal of anti-TNF drugs.43,44
Immune-mediated DiseasesAn emerging issue related to the administration of anti-TNF therapy is the development of immune mediated diseases, manifested mainly on the skin. The first reported in the literature was the appearance of “de novo” psoriasis in patients with various inflammatory joint diseases, with no history of psoriasis, who developed skin lesions after initiation of anti-TNF7 therapy. This reaction is considered as paradoxical in view of the demonstrated efficacy and indication of anti-TNF for treatment of PsA47 and psoriasis.48 Subsequently, there have been other cases of psoriasis not only “de novo”,49 but of exacerbations of psoriasis after prior anti-TNF treatment, rather than improvement.50,51 This has been, so far, the most frequently reported immune-mediated disease after anti-TNF treatment, which has focused attention on this type of skin effects.
Next, we describe the most common immune-mediated skin diseases related to anti-TNF treatment.
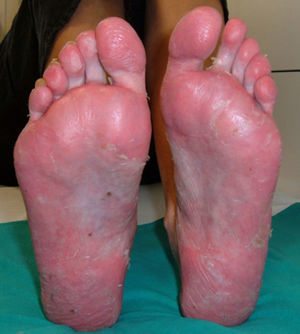
PsoriasisAlthough the development of psoriasis has been reported after administration of various drugs, such as antimalarials or betablockers,52 in the case of anti-TNF the paradox is that the skin lesions of psoriasis develop after administration of a drug indicated for its treatment. Today over 200 cases of psoriasis induced by anti-TNF53 have been reported, although there are probably many more unreported cases. In fact, although the prevalence varies in different studies, it ranges from 0.6% to 5.3% depending on the patient population,7,9,10,49 although in general, it is considered higher than in controls.49 Most cases have been reported in patients with RA,10,49,53,54 although cases have been reported in almost all inflammatory diseases, such as SA, inflammatory bowel disease, PsA, juvenile idiopathic arthritis, psoriasis, Behçet's disease or SAPHO syndrome, among others.7,50,54–56 The development of these skin lesions can occur at any time during treatment, from days to years after onset, although the average latency time is about 10 months after initiation of therapy.7,10,51,55,57 We found no differences in age or gender of patients and no predisposing risk factors for the development of this adverse effect. Moreover, concomitant treatment with other immunosuppressive drugs for psoriasis has not proven effective as a preventive measure for the development of this effect.53 In general, the majority of patients who develop this adverse effect have presented a good response to biological therapy, without the appearance of the skin lesion adversely affecting drug efficacy. From the standpoint of the skin, there have been 3 main morphologies described for the, each with different frequency: pustular psoriasis (56%), signs of psoriasis (50%) and guttate (12%),53 although there may be other skin presentations such as erythrodermic psoriasis or inverted psoriasis, among others, with less frequency.58 However, one of the characteristic lesions frequently associated with anti-TNF therapy is the development of palmoplantar pustulosis (Fig. 1). It is characterized by the development of small blisters of sterile content, located on the palms and soles, with burning and itching, which is associated with erythema, and hyperkeratosis. It is also estimated that 15% of patients have more than one type of lesion, without statistical correlation between the type of skin injury and the underlying disease.53 This has also been reported with nail changes typical of psoriasis such as onycholysis, discolouring and pitting.53
In addition to the 3 ‘classic’ anti-TNFs, cases of certolizumab induced psoriasis have been described,59,60 so this is considered a “class” adverse effect for these drugs. However, some authors found a higher incidence of this effect in connection with the administration of monoclonal antibodies such as infliximab50 or adalimumab.49 Likewise, other authors suggest that monoclonal antibodies would be more related to the development of “de novo” psoriasis, while etanercept would be associated primarily with exacerbation of prior psoriasis61 (Fig. 2). This effect could be due to the fact that in individuals with psoriasis, large oscillations of TNF are not required to trigger an outbreak of psoriasis, though a severe reduction of TNF would be required to induce psoriasis ‘de novo’ in predisposed individuals, an effect that would be more frequent with monoclonal antibodies, more potent inhibitors of TNF.
Initially, some authors considered that these skin lesions could correspond more to a hypersensitivity reaction than to true psoriasis, but skin biopsies of selected cases found histological lesions identical to those of individuals with idiopathic psoriasis,10,55 including epidermal hyperplasia, parakeratosis, epidermal lymphocytic infiltrates, dilation of capillaries and intradermal pustulosis,53 in contrast to the histology of patients with psoriasis induced by other treatments,62 suggesting that the mechanism of action of the two processes is similar or identical. Likewise, other authors considered that it could be a misdiagnosis and that they were in fact patients with PsA, as arthritis may precede psoriasis in 15% of patients. However, the majority of cases have been reported in patients with a definite diagnosis of RA.49,54 Moreover, the coexistence of these two diseases, RA and psoriasis, is extremely rare, having been described in 0.2% of patients, while the incidence of psoriasis induced by anti-TNF occurs in 3% in patients with spondyloarthropathies and between 2.3 and 5% in patients with RA,7,10,55 a much higher incidence than expected.
Although its pathophysiology is not known exactly, several hypotheses have been postulated that explain the development of paradoxical psoriasis. One of the most widely accepted comes from the relationship between TNF and interferon α type 1, the latter a factor that plays an important role in the pathogenesis of psoriasis through its synthesis by dermal plasmacytoid dendritic cells. Under normal conditions, TNF would produce an inhibition of the synthesis of interferon by these cells. After treatment with anti-TNF drugs it could produce an imbalance in cytokines, inducing local production of interferon in some patients, triggering an outbreak of psoriasis.55 Supporting this hypothesis there is evidence of an increase in the expression of interferon in the skin lesions of these patients compared with patients suffering from idiopathic psoriasis.55 Other authors have observed that in these patients there is an increased number of peripheral T cells that express the CXCR363 receptor, which promotes the infiltration of autoreactive T cells into the skin.7,64 It appears that the induction of this receptor would be mediated by interferon-α.55 This increase in peripheral T cell population was observed in RA patients after treatment with anti-TNF, and is thought to be secondary to a decrease in the traffic of these cells to areas previously swollen, as joints. However, this increase in peripheral T cells was confirmed in further studies and in diseases other than RA.63
In the case of pustules, a common injury in this type of adverse effect and characteristically involving palms and soles, these differ from plaque psoriasis, not only clinically but also inmunohistochemically.55 As is well known, TNF contributes to the development of psoriasis plaques, and in palmoplantar pustulosis a decrease in the expression level of the eccrine palmar sweat glands57 has been found. It has also recently been described both as cases of palmoplantar pustulosis lesions and as a worsening of baseline psoriasis induced by other non-anti-TNF65,66 biologics, suggesting that in the pathogenesis of psoriasis, the true mechanism of action could affect multiple cytokines such as IL-17 or IL-22 and not only due to a blockade of TNF.
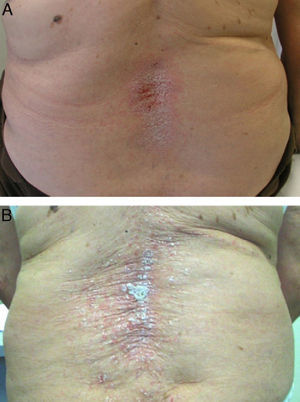
Regarding the treatment of these skin lesions, almost all authors agree that biological drug withdrawal is followed by their improvement or resolution49,54 (Fig. 2A), and some authors suggest the desirability of withdrawing the anti TNF.51 It is estimated that following the withdrawal of the drug, the lesions persist only in approximately 5% of the cases.53 Discontinuation rates vary in different biological studies, 14.8%10 and 19.5%67 to 32%50 and 39% of patients. However, unlike other pharmacological causes producing psoriasis, anti-TNF withdrawal may cause a disease flare, which may be more severe than the skin reaction itself. Furthermore, due to the exacerbation of underlying disease after withdrawal of anti-TNF, many patients require a rebooting of the biological treatment with, as observed in many cases, a recurrence of skin lesions (Fig. 2B), which sometimes can successfully respond to treatment while continuing the biological treatment.7,9,54,55,57,64,68 This would suggest that, in many cases, a proper treatment of skin lesions might allow the maintenance of biological therapy for disease control. Thus, in a recently published study between 66%53 and 79%56 of patients continued anti-TNF treatment and concomitant treatments for psoriasis, and many patients were able to be treated conservatively without removing the biological. It was also noted that 26% of the patients who maintained anti-TNF treatment achieved complete resolution, and a third partial resolution of lesions.
Since this cutaneous adverse effect may differ in severity, some authors have suggested a treatment algorithm based on the number of patients and review of cases published in the literature.56 First, refer patients suspected of psoriasis induced by anti-TNF to dermatologists for evaluation, diagnostic and histologic confirmation in those cases requiring exclusion of other skin lesions. Also exclude a bacterial or viral infection. After confirming the diagnosis of psoriasis, the extent and tolerability of lesions should be assessed. Thus, in patients with severe psoriasis, erythrodermic varieties, lesions affecting the quality of life of patients and lesions showing an increase in size and severity after each infusion or injection treatment, it is recommended to discontinue biological therapy and perform intensive treatment of skin lesions. In those patients in whom skin lesions affect <5% of body area, topical treatments including corticosteroids are recommended, among others, and if no improvement is seen in 2–3 weeks, consider treatment with ultraviolet light therapy or methotrexate. In patients with lesions affecting >5% of the body surface or showing plantar pustulosis, topical treatment and phototherapy are advised, and those who do not respond should consider systemic treatment with methotrexate, retinoids or cyclosporine. If no response to these treatments is seen then it is advisable to consider the discontinuation of biologic therapy and switching to a non-biological anti-TNF, if feasible. In summary, in most cases this adverse effect would not require discontinuation of biological treatment, but we recommend aggressive treatment of skin lesions and only consider changes in the anti-TNF if the lesions do not respond to standard treatment of psoriasis.
Cutaneous Lupus ErythematosusTreatment with anti-TNF has been associated with the development of various autoantibodies such as antinuclear, anti-DNA, anticardiolipin or anti-histone antibodies, with variable frequencies depending on the studies.69,70 However, the occurrence of these autoantibodies usually presents without symptoms and clinical development of lupus erythematosus is extremely infrequent.71 However, there are some cases of anti-TNF induced lupus in the literature67,72–76 where skin lesions are common and, exceptionally, neurological impairment. In general, skin lesions usually present as a maculopapular erythematous, pruritic rash, usually affecting sensitive areas like the face, upper extremities and upper trunk. It can be observed in different pathologies and is frequently described in association to infliximab and etanercept,73,77,78 and there are cases described associated to adalimumab.79 It affects women predominantly, in the fifth decade of life, and the onset of symptoms can vary widely from one month to years after initiation of anti-TNF. In these cases, antinuclear and anti-DNA antibodies are frequently positive, but others such as anti-histone antibodies are usually negative. Various pathogenic mechanisms of this syndrome as a result of the effects of anti-TNF have been suggested, such as the induction of apoptosis, immunosuppression or autoimmunity humoral.72,78 Other authors suggest an important role for type 1 interferon α in the pathogenesis of lupus, and it has been postulated that a dysregulation of interferon α could lead to the development of antinuclear antibodies and lupus-like syndrome in patients with anti-TNF treatment.80 In most of these cases the discontinuation of the drug is followed by complete resolution, although occasionally patients may require treatment with corticosteroids, antimalarials, or immunosuppressants, according to the severity. If present biological therapy must be reconsidered due to the possibility of reactivation of the disease, the cutaneous adverse effect would not be an absolute contraindication to further treatment with other anti-TNF alternatives.72
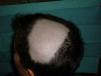
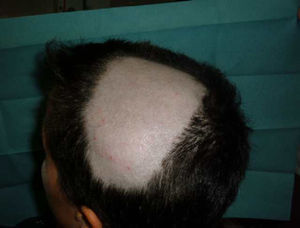
Alopecia AreataThere have also been reports of alopecia areata associated with anti-TNF treatment.9,67,76,81–89 Alopecia areata is considered an autoimmune disease mediated by T lymphocytes, characterized by hair loss in patches, generally circular, mainly affecting the scalp81,82,84,89 (Fig. 3), but which sometimes can spread and affect the entire cranium (alopecia totalis)83,87 and even the entire body (alopecia universalis).85,86 The time of onset of alopecia areata may vary from 1 to 2 days84 up to 24 months86 after the start of biological therapy, and a history of alopecia areata is found in almost half of patients. In approximately half of the cases described, the anti-TNF81,84–86,89 was discontinued, despite which half of them presented progression of alopecia.81,85,86 In most cases the patients were treated with topical corticosteroids. In the cases published, alopecia areata is not accompanied by other systemic manifestation but the condition is limited to the skin. In cases that have been studied, no abnormalities were found in other tests, including autoimmune changes, discarding the development of a lupus-like84 syndrome. The fact that there have been reports of alopecia areata “de novo” in subjects treated with etanercept and that many cases have been reported with monoclonal antibodies, suggest a greater association of these drugs with this side effect. Its pathogenic mechanism is not well known, and in fact, TNF-α is associated with a development of alopecia because of a demonstrated in vitro inhibition of hair growth. However, treatment with anti-TNF drugs has not proven effective in the treatment of alopecia, as demonstrated in a clinical trial with etanercept.90 Some authors suggest that the blockade produced by anti-TNF drugs may cause dysregulation of cytokines such as interferon-α and activation of autoreactive T cells resulting in the development of alopecia areata in predisposed individuals.85
Cutaneous VasculitisThe development of cutaneous vasculitis associated with anti-TNF treatment has also been described, mainly leukocytoclastic and, less frequently, necrotizing vasculitis75,76,91–93; the characteristic skin lesion is palpable purpura, although other lesions have been described such as ulcers, nodules or rash. Occasionally, skin lesions begin at the site of injection of the drug and spread and affect other skin areas supporting a possible mechanism of antigen mediated hypersensitivity vasculitis. In most cases there is an improvement of the lesions after discontinuation of the anti-TNF drug.
VitiligoAnother immune-mediated skin lesion reported after anti-TNF treatment is the development of vitiligo. This disease, autoimmune in nature, is characterized by hypopigmented skin lesions due to a partial or total loss of pigment in melanocytes. However, unlike the lesions of psoriasis, the skin lesion is rare and few cases have been described in the literature, most associated to infliximab67,76,94,95; also, the development of vitiligo has been associated with other autoimmune diseases such as pernicious anemia, Addison's disease or autoimmune thyroiditis, so in these patients it is important to rule out their development.
Relapsing PolychondritisRelapsing polychondritis is an immune-mediated disease, rare and of unknown etiology, characterized by inflammation and progressive destruction of cartilage, especially affecting the ears, nose, joints and tracheobronchial tree. So far only 2 cases have been reported to be associated with anti-TNF96 therapy, both with full recovery of the inflammatory destruction of cartilage and not affected after suspension of anti-TNF.
Polymyositis/DermatomyositisIsolated cases of polymyositis/dermatomyositis (PM/DM) associated with treatment with anti-TNF97,98 have been described, mostly in patients with RA, with complete recovery after removal of the agent and treatment with glucocorticoids. Many of these patients had anti-Jo-1 antibodies before the start of biological therapy, suggesting a correlation between the onset of PM/DM with anti-Jo-1, and anti-TNF treatment for their primary disease.98 As in systemic lupus, psoriasis or alopecia areata, some authors have associated dermatomyositis with dysregulation in the synthesis of interferon 1α.99
Localized Scleroderma (Morphea)Another disease associated with anti-TNF is morphea or localized scleroderma, characterized by fibrosis of the skin and subcutaneous tissue. Although it can be triggered or exacerbated by drugs such as isoniazid, antimalarials or penicillamine and bleomycin, a case triggered after biological100–102 therapy has been described, usually with a good outcome after discontinuation of the drug and without progression. Several hypotheses have been proposed regarding its pathogenesis including the effect of TNF blockade on transforming growth factor beta 1 (TGF-β1), a profibrotic cytokine,103 or any increase of activated T cells, with a predominance of Th2 cytokines, which correlate with extent of injury and fibrosis in morphea over Th1 cytokines, inducing, therefore, the development of morphea.104
Other Immune-mediated Skin DiseasesBesides those described above, there have been reports of immune-mediated skin diseases associated with anti-TNF treatment such as granuloma anularis,76,105 lichen or lichenoid reactions,10,67,76,91,106,107 and pemphigus,108–110 suggesting the skin as one of the main targets of these drugs.
Overall, the patterns for the development of various autoimmune diseases often overlap and increased the likelihood of developing a second autoimmune disease after diagnosis of the first has been widely accepted. In this case, the anti-TNF and possibly all biological drugs have the potential to disrupt the delicate balance of cytokines or chemokines and promote, in an individual with genetic predisposition, the development of these skin-dominated autoimmune diseases. Although it is sometimes rare, findings reveal different immune-mediated diseases induced by anti-TNF with generally good outcome after drug withdrawal, unlike the course that usually follows primary autoimmune disease.
In conclusion, the development of skin lesions may be an adverse effect that is becoming more common due to the widespread use of anti-TNF drugs in different diseases. The type of adverse effect, wherein the classical skin lesions described following administration of treatment to other types of more relevant skin lesions include not only infections and malignancies, but also emerging adverse events such as autoimmune disease with a predominantly skin affection. The therapeutic advantages of anti-TNF exceed, in most cases, the risk of developing this side effect, but some cases require the withdrawal of the drug. Doctors and patients should be aware of this potential adverse effect for an early and correct diagnosis and appropriate treatment leading to their resolution, something that requires, in many cases, the involvement of the dermatologist.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Hernández MV, et al. Lesiones cutáneas y terapia biológica con antagonistas del factor de necrosis tumoral. Reumatol Clin. 2013;9:53–61.