The treatment of noninfectious uveitis includes steroids and immunomodulatory drugs, the use of which has increased in the last few years, and the options have been enriched with the development of new treatments. However, clear therapeutic guidelines and protocols have not been developed. The purpose is to analyse the response to the drugs used and the characteristics of the patients treated at a multidisciplinary uveitis clinic.
Material and methodsObservational and retrospective study of the patients attended to from January 2012 to December 2015. Infectious, posttraumatic and postoperative uveitis, as well as masquerade syndrome, were excluded.
ResultsTwo hundred six patients were included. Overall, 58.80% had uveitis without association of systemic disease, mostly idiopathic uveitis, and 35.65% had uveitis with systemic involvement, mainly related to spondyloarthritis. Uveitis without systemic association and anterior uveitis achieved disease control with local treatment more frequently than others (P=.002 and P<.001, respectively). In all, 49.76% of the patients required systemic treatment. Among those treated with immunomodulators, 53.26% needed a second drug and 31.52% needed a third drug. Women required immunomodulators more often than men (P=.042). Methotrexate was the most widely used immunomodulator. Posterior uveitis responded less favourably to the second immunomodulator than anterior uveitis (P=.006).
ConclusionsAlmost half of the patients needed an immunomodulatory drug and some of them required successive drug changes. Intermediate uveitis was the most treatment-refractory uveitis.
En el tratamiento de las uveítis no infecciosas se emplean corticoides y fármacos inmunomoduladores. Su uso ha aumentado en los últimos años y se ha enriquecido con la aparición de nuevos tratamientos. Sin embargo, no existen guías ni protocolos claros de actuación. El objetivo es analizar la respuesta a los fármacos empleados y las características de los pacientes atendidos en una consulta multidisciplinaria de uveítis.
Material y métodosEstudio observacional retrospectivo de los pacientes atendidos desde enero de 2012 hasta diciembre de 2015. Se excluyen las uveítis infecciosas, posquirúrgicas, postraumáticas y los síndromes de enmascaramiento.
ResultadosSe incluyeron 216 pacientes. El 58,80% son uveítis sin afectación sistémica, la mayoría idiopáticas, y el 35,65% uveítis con afectación sistémica, asociadas principalmente a espondiloartritis. Las uveítis sin afectación sistémica y las uveítis anteriores se controlaron mejor que el resto con tratamiento local (p=0,002 y p<0,001, respectivamente). El 49,76% de los pacientes requirió tratamiento sistémico. De los pacientes tratados con inmunomoduladores, el 53,26% precisó un segundo fármaco y el 31,52%, un tercero. Las mujeres necesitaron inmunomoduladores con más frecuencia que los varones (p=0,042). El inmunomodulador más empleado fue metotrexato. Las uveítis posteriores respondieron al segundo inmunomodulador peor que las anteriores (p=0,006).
ConclusionesCasi la mitad de los pacientes necesitaron un fármaco inmunomodulador y algunos precisaron varios cambios sucesivos de fármaco. Las uveítis intermedias resultaron las más refractarias al tratamiento.
Uveitis is inflammation of the middle layer of the eye (iris, ciliary body and choroid), although other structures (sclera, retina, optic nerve) can also be affected. The condition can be of endogenous origin, associated or otherwise with systemic diseases, or exogenous.1 Classification is based on the anatomical site of the inflammation, its time course and aetiology.2–4 The incidence of uveitis in developed countries is 17–52 cases per 100,000 inhabitants per year, with a prevalence of 38–714 cases per 100,000 inhabitants.5–7 The incidence is similar in males and females, although more prevalent in females.6,8
These conditions are significant in that they cause notable morbidity, and principally affect young adults,1,8 with major socioeconomic consequences.1,9 In fact, they are the fourth cause of blindness among the active population, and the second cause of potentially treatable and preventable blindness in the developed countries.9 Loss of vision is mainly due to the development of cystic macular oedema.10
Non-infectious uveitis is usually treated initially with topical corticosteroids, which are particularly effective for anterior uveitis.11 However, it is occasionally necessary to resort to systemic therapy, both oral corticosteroids and immunomodulatory drugs,12,13 which are used for cases that are refractory to corticosteroids or to minimise their side effects,12,14 and it is often necessary to use several of them successively or in combination.1,12 However, possibly due to the heterogeneity of presentation, the emergence of new drugs, and the aetiology and prognosis of this set of diseases, there are still no clear action guidelines or protocols. Multidisciplinary uveitis clinics have emerged to improve the approach to these patients, where ophthalmologists and rheumatologists interact to contribute within their area of knowledge. This should result in better diagnosis and treatment and promote advances in this area.15–17
The aims of this paper were to analyse the features of the patients attending our hospital's multidisciplinary uveitis clinic, and to study the use of immunomodulators in treating the condition, to establish the level of response according to the type of uveitis, and thus acquire the knowledge to improve the treatment and prognosis of our patients.
Material and MethodsA retrospective, observational study was undertaken of the patients attended by the rheumatologist in the multidisciplinary uveitis clinic of a tertiary referral hospital from January 2012 to December 2015. Infectious, postsurgical, posttraumatic, and masquerading syndromes were excluded. The study was approved by the hospital's Research Ethics Committee (PI-2246).
We gathered the demographic data, age of onset of the ocular symptoms, relevant personal and family history, details of the ophthalmological examination, and the results of complementary tests.
The types of uveitis were classified according to their aetiology (without systemic involvement, with systemic involvement), anatomical site (anterior, intermediate, posterior, panuveitis), laterality (unilateral, bilateral), and course (acute, recurrent, chronic).2–4
Data relating to the treatments given were also collected: local (topical eye drops, peri-ocular and intra-ocular injections), and systemic (oral corticosteroids, immunomodulatory drugs). The response to treatment was also recorded: lack of response or improvement in the eye disease according to the judgement of the ophthalmologist, based on the usual response parameters according to the pattern of uveitis (anterior Tyndall, vitreous Tyndall, macular oedema, chorioretinitis, vasculitis, etc.). Intolerance or toxicity were also recorded, and discontinuation of treatment or lack of follow-up. Immunomodulatory treatments prescribed for other manifestations of the associated systemic disease were excluded from the analysis, and those started prior to the uveitis consultation.
Statistical AnalysisThe data were analysed using SPSS 15.0. P values <.05 were considered statistically significant.
The quantitative variables were analysed using the Student's t-test for comparing the means of two independent samples. A test for normal distribution was performed to study the applicability of this test (Komogorov–Smirnov test for samples of n>30, and Shapiro–Wilk test for samples of n≤30), and after confirming their normal behaviour, Levene's test was used to confirm the homogeneity of variances.
The qualitative variables were analysed using the Z test for comparing percentages, and Pearson's chi-squared association test. Fisher's exact test was used when the number of sample events or the frequencies expected on the tables exceeded five.
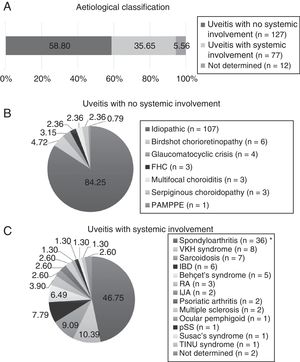
ResultsDemographic Features and Involvement PatternsTwo hundred and sixteen patients were included, of which 134 (62.04%) were female. The mean age at the start of follow-up was 50.04±15.93 years. According to the aetiology (Fig. 1), the uveitis types without systemic involvement were the most common (58.80%), and were idiopathic in the majority (84.25%). The types of uveitis associated with systemic inflammatory disease comprised 35.65%, and were principally associated with spondyloarthritis (59.74%), including ankylosing spondylitis, non-radiographic axial spondyloarthritis, peripheral spondyloarthritis, juvenile idiopathic arthritis, psoriatic arthritis, and inflammatory bowel disease. A small percentage could not be classified aetiologically due to a lack of data (12 patients, 5.56%).
Aetiological classification. IJA: idiopathic juvenile arthritis; RA: rheumatoid arthritis; FHC: Fuchs’ heterochromic cyclitis; IBD: inflammatory bowel disease; MS: multiple sclerosis; PAMPPE: posterior acute multifocal placoid pigment epitheliopathy; pSS: primary Sjögren's syndrome; TINU: tubulointerstitial nephritis and uveitis; VKH: Vogt-Koyanagi-Harada.* Includes ankylosing spondylitis, axial spondylitis, non-radiographic spondyloarthritis and peripheral spondyloarthritis.
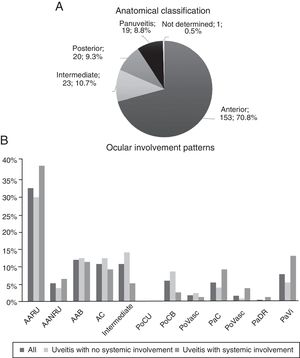
The graphs of Fig. 2 describe the anatomical site and the involvement patterns. Anterior types predominate (70.83%), and the principal involvement pattern was acute recurrent unilateral anterior uveitis (32.41%).
Anatomical classification and ocular involvement patterns. BAA: bilateral acute anterior; AUNRA: acute unilateral non-recurrent anterior; AARU: acute unilateral recurrent anterior; CA: chronic anterior; PaC: panuveitis with chorioretinitis; PaRD: panuveitis with exudative retinal detachment; PaVasc: panuveitis with retinal vasculitis; PaVi: panuveitis with vitritis; PoCB: posterior with bilateral chorioretinitis; PoCU: posterior with unilateral chorioretinitis; PoVasc: posterior with retinal vasculitis.
The features of the patients were studied according to the treatment received, either local or systemic (including corticosteroids and immunomodulatory drugs) (Table 1). Nine patients were excluded from the analysis who had previously taken immunomodulators for their baseline disease. Of the 207 patients studied, 103 (49.76%) received systemic treatment, and 104 (50.24%), local treatment. The aforementioned 12 patients with no aetiological classification were included in the latter group.
Type of Treatment Requireda
| Local treatmentn=104 | Systemic treatment(oral corticosteroids or immunomodulators)n=103 | P value | |
|---|---|---|---|
| Age – yearsb | 53.25±16.77 | 47.15±14.97 | .008 |
| Sex | .339 | ||
| Females | 62 (59.62) | 68 (66.02) | |
| Aetiology | .002 | ||
| Without/with systemic involvement | 70/22(76.09%/23.91) | 57/46(55.34%/44.66) | |
| Anatomy | |||
| Anterior | 91 (87.50) | 53 (51.96) | <.001 |
| Intermediate | 10 (9.62) | 13 (12.75) | .476 |
| Posterior | 0 | 20 (19.61) | <.001 |
| Panuveitis | 3 (2.88) | 16 (15.69) | .001 |
| Anatomy and aetiology | |||
| Without systemic involvement | |||
| Anterior | 59 (64.13) | 27 (26.47) | <.001 |
| Intermediate | 9 (9.78) | 9 (8.82) | .819 |
| Posterior | 0 | 15 (14.71) | <.001 |
| Panuveitis | 2 (2.17) | 6 (5.88) | .284 |
| With systemic involvement | |||
| Anterior | 21 (22.83) | 26 (25.49) | .665 |
| Intermediate | 0 | 4 (3.92) | .123 |
| Posterior | 0 | 5 (4.90) | .061 |
| Panuveitis | 1 (1.09) | 10 (9.80) | .011 |
The values in percentages are in brackets. The statistically significant results are highlighted in bold, P<.05.
Differences in the age at the start of follow-up were found between both treatment groups, the patients who required systemic treatment were younger (P=.008). According to the aetiology, the types of uveitis with extraocular involvement required more systemic treatment (P=.002). Likewise, differences were found in the response to treatment according to the anatomical pattern, P<.001 for anterior and posterior uveitis, and P=.001 for panuveitis. In fact, only 39.85% of the anterior types required systemic treatment, while 100.00% and 84.21% of the cases of posterior uveitis and panuveitus respectively required systemic treatment: 59.09% of the intermediate uveitis cases required systemic treatment.
Corticosteroid TreatmentOf the 103 patients who required systemic treatment, 69 (66.99%) received oral corticosteroids and, of those, 11 (10.68%) were controlled without the need for immunomodulatory drugs. There were no statistically significant differences between the patients who required oral corticosteroids and those who did not.
Treatment With Immunomodulatory DrugsNinety-two patients received a first immunomodulatory drug. Women predominated in this patient group (64 patients, 69.57%). Of the 92 patients, 49 (53.26%) required a second immunomodulatory drug, 29 (31.52%) required a third, and 14 (15.22%) needed between four and six drugs (Table 2). All the biological treatments were anti-TNF (infliximab or adalimumab), administered to 4 patients with spondyloarthritis, 3 with Vogt–Koyanagi–Harada (VKH) syndrome, 3 with Birdshot disease, 2 with Behçet's syndrome, 2 with sarcoidosis, one with juvenile idiopathic arthritis, one with inflammatory bowel disease, and 4 with no systemic involvement (two with panuveitis and two with chronic anterior uveitis).
Treatment With Immunomodulators. Drugs Used and Response to Them According to Aetiology and Anatomical Pattern.
| Aetiology | Anatomy | ||||||
|---|---|---|---|---|---|---|---|
| Without systemic involvement | With systemic involvement | Anterior | Intermediate | Posterior | Panuveitis | ||
| 1st IMM (n) | 92 | 49 | 43 | 48 | 12 | 16 | 15 |
| Response (n) | Yes | 22 | 20 | 26 | 4 | 6 | 5 |
| No | 19 | 17 | 18 | 6 | 7 | 5 | |
| AE | 7 | 6 | 4 | 2 | 2 | 5 | |
| LF | 1 | 0 | 0 | 0 | 1 | 0 | |
| MTX: 24 | SSZ: 14 | SSZ: 22 | MTX: 6 | AZA: 6 | MTX: 8 | ||
| CyA: 11 | MTX: 13 | MTX: 18 | CyA: 4 | CyA: 5 | CyA: 4 | ||
| SSZ: 9 | AZA: 6 | aTNF: 3 | aTNF: 1 | MTX: 5 | AZA: 3 | ||
| AZA: 4 | CyA: 3 | LEF: 2 | AZA: 1 | ||||
| Other: 1 | Other: 7 | Other: 3 | |||||
| 2nd IMM (n) | 49 | 26 | 23 | 22 | 8 | 9 | 10 |
| Response (n) | Yes | 8 | 8 | 10 | 2 | 0 | 4 |
| No | 8 | 11 | 8 | 3 | 4 | 4 | |
| AE | 8 | 2 | 2 | 1 | 5 | 2 | |
| LF | 2 | 2 | 2 | 2 | 0 | 0 | |
| AZA: 9 | aTNF: 9 | MTX: 9 | AZA: 6 | CyA: 3 | MTX: 5 | ||
| MTX: 9 | MTX: 8 | aTNF: 7 | MTX: 2 | aTNF: 2 | aTNF: 3 | ||
| aTNF: 3 | AZA: 4 | AZA: 3 | AZA: 2 | AZA: 2 | |||
| CyA: 3 | Other: 2 | SSZ: 3 | Other: 2 | ||||
| Other: 2 | |||||||
| 3rd IMM (n) | 29 | 16 | 13 | 10 | 4 | 9 | 6 |
| Response (n) | Yes | 9 | 4 | 5 | 1 | 6 | 1 |
| No | 3 | 2 | 2 | 0 | 2 | 1 | |
| AE | 4 | 5 | 3 | 2 | 1 | 3 | |
| LF | 0 | 2 | 0 | 1 | 0 | 1 | |
| AZA: 7 | MTX: 5 | MTX: 3 | AZA: 1 | AZA: 4 | aTNF: 3 | ||
| aTNF: 3 | aTNF: 3 | AZA: 2 | CIC: 1 | aTNF: 2 | AZA: 1 | ||
| Other: 6 | CyA: 2 | Other: 5 | CyA: 1 | Other: 3 | MTX: 1 | ||
| Other: 3 | MTX: 1 | SSZ: 1 | |||||
aTNF: anti-TNF; AZA: azathioprine; CIC: cyclophosphamide; CyA: cyclosporine; AE: adverse effect; IMM: immunomodulator; LEF: leflunomide; MTX: methotrexate; LF: lost to follow-up; SSZ: sulfasalazine.
Table 2 also details the drugs used as first, second and third lines of treatment, and the response achieved according to aetiology and anatomical site. Methotrexate is used most as a first option. Methotrexate and sulfasalazine are prescribed in equal measure for uveitis with systemic involvement; sulfasalazine is used more for anterior uveitis and azathioprine for posterior uveitis. Methotrexate is still the drug used most as a second immunomodulator, but azathioprine and infliximab are also commonly used.
Table 3 shows the proportion of patients controlled with the first three immunomodulators. The males responded better than the females to the first immunomodulator, although this difference did not achieve statistical significance (P=.055). There were no significant differences in the response to the successive drugs in terms of age, and the presence or otherwise of systemic involvement.
Proportion of Patients Who Responded Favourably to Immunomodulatorsa
| Controlled with1 drug | Controlled with≤2 drugs | Controlled with≤3 drugs | |
|---|---|---|---|
| Sex | |||
| Female (n=64) | 25 (39.06)b | 38 (59.38) | 47 (73.44) |
| Male (n=28) | 17 (60.71) | 20 (71.43) | 24 (85.71) |
| Age | |||
| ≤40 years (n=35) | 15 (42.86) | 20 (57.14) | 24 (68.57) |
| 40–60 years (n=32) | 14 (43.75) | 20 (62.50) | 27 (84.38) |
| >60 years (n=21) | 11 (52.38) | 15 (71.43) | 17 (80.95) |
| Aetiology | |||
| Without systemic involvement (n=49) | 22 (44.90) | 30 (61.23) | 39 (79.59) |
| With systemic involvement (n=43) | 20 (46.51) | 28 (65.12) | 32 (74.42) |
| Anatomy | |||
| Anterior (n=48) | 26 (54.17) | 36 (75.00)c | 41 (85.42)d |
| Intermediate (n=12) | 4 (33.33) | 6 (50.00) | 7 (58.33) |
| Posterior (n=16) | 6 (37.50) | 6 (37.50) | 12 (75.00) |
| Panuveitis (n=15) | 5 (33.33) | 9 (60.00) | 10 (66.67) |
The values in percentages are in brackets.
According to anatomical site, 48 of the 153 patients with anterior uveitis (31.37%) required immunomodulatory drugs, 85.42% were controlled with the first, second or third drug. Of the intermediate uveitis cases, 12 of 23 (52.17%) required immunomodulators, and only 58.33% were controlled with the three first drugs. Of the posterior uveitis cases, 16 of 20 (80.00%) required this treatment, with a favourable response in 75.00% with the administration of one to three drugs. Finally, 15 out of 19 patients with panuveitis (78.95%) were treated with immunomodulators, and 66.67% were controlled with the three first drugs.
Of the uveitis patients with systemic disease who needed immunomodulatory treatment, 25 were anterior, 3 intermediate, 5 posterior and 9 panuveitis.
On analysing the patients who were controlled with one or two immunomodulators, the cases of posterior uveitis responded more poorly than the anterior cases (P=.006). The intermediate uveitis cases also responded more poorly than the anterior cases, with a difference that was just statistically significant (P=.051).
DiscussionThe most common non-infectious uveitis cases in our clinic are those with no idiopathic systemic involvement, and those associated with spondylarthritis, similar to that described in other studies.15,17–19 The high percentage of idiopathic uveitis in our sample compared to other reviews performed in our country is because we excluded infectious, postsurgical, posttraumatic uveitis and masquerading syndromes, which appear in high percentages in other series.20 There are other areas where cases of uveitis associated with Behçet's syndrome, sarcoidosis, and VKH are more prevalent.8,21
According to anatomical site, anterior forms of uveitis were most common and, among them, the acute recurring types. The intermediate and posterior types, and panuveitis were less common, similar to other studies.8,15,17–19 However, there are populations where the posterior types and panuveitis are more frequent than the intermediate,8,18,19 probably due to a greater prevalence in these areas of uveitis cases with posterior involvement, such as those associated with Behçet's and VKH syndromes, and because infectious uveitis was not excluded in these studies.
Anterior uveitis was statistically significantly in the majority among the patients controlled with local treatment, as is also reflected in other papers.11,14 According to the aetiology, it was observed that uveitis with no systemic involvement is controlled better, which is consistent with the above, since the majority are anterior. Therefore, the patients with uveitis associated with systemic disease, posterior uveitis or panuveitis are more likely to need systemic treatment. When we analyse the anatomical pattern and the aetiology (Table 1), in the cases with systemic involvement the anatomical pattern has little influence, and generally requires more systemic treatment.
It is also worth highlighting that the patients who required systemic treatment were younger than those controlled with local treatment. This might be explained by the asymmetric distribution of the different types of uveitis according to age. In our clinic, the patients aged over 50 had more uveitis with no extraocular association, anterior uveitis and panuveitis, and those under 50 were diagnosed with more uveitis associated with systemic disease, intermediate and posterior uveitis. We think that these findings are interesting, but we have found no information in the literature in this regard.
Of the patients given systemic treatment, the females were controlled less well than the males, and more of them required immunomodulatory drugs. This might be explained by the greater frequency of males with acute, non-recurrent uveitis.
The cases of posterior uveitis in our clinic required oral corticosteroids and immunomodulatory drugs in equal measure, unlike other studies, where the majority are controlled with oral corticosteroids.1 This might be due to the change in treatment guidelines in recent years, with the reduction of systemic corticosteroid doses, and the earlier introduction of immunomodulators. Moreover, 45% of patients diagnosed with uveitis in our clinic, and who had not received previous immunosuppressive therapy for their baseline disease, started immunomodulatory treatment. This information is different to other papers,22 where the need for immunomodulators is less (14%), probably due to the inclusion of infectious, postsurgical and posttraumatic uveitis in these studies.
Methotrexate is the drug most used in our clinic, as in other studies, probably due to its good efficacy-cost-toxicity ratio.12,23 Cyclosporine is the second most used drug for uveitis with no systemic involvement, as in other papers,24,25 especially for the intermediate and posterior types. Sulfasalazine is important for uveitis associated with systemic disease and anterior uveitis, due to its good results for anterior uveitis associated with spondyloarthritis.26
The biological treatments are drugs with demonstrated efficacy as well.1,12,27 The most used drugs in our patients were infliximab and adalimumab, which are also the most used in other series in recent papers.12,13,28
With regard to change of immunomodulator, the first controlled ocular inflammation in 45% of our patients, which is similar to other studies.29 However, this varies according to the type of uveitis. According to sex, the women responded more poorly to the first drug, and required a second in 60% of cases, compared to 40% of the males. This, as we mentioned, is probably due to the differences in aetiology and the involvement pattern detected.
We also found differences in terms of anatomical site. Seventy-five percent of the anterior uveitis cases were controlled with a first or second drug, while more than half the posterior cases required at least three. We also observed a poorer response in the intermediate uveitis compared to the anterior cases. Forty percent of the intermediate uveitis cases required a fourth immunomodulator to control the disease, compared to 15% of the anterior cases. We were not able to contrast these data in the literature because there are no studies that analyse the response of these uveitis cases to the successive immunomodulatory drugs. However, the greater severity and poorer prognosis of posterior uveitis is known.1,9
A recent study evaluated the discontinuation rate of the various immunosuppressive drugs in 110 patients, and showed a retention rate for these drugs at one year and at 10 years of 74% and 16% respectively. The reasons for discontinuation were inefficacy or adverse effects in almost half the cases, similar to our frequency, and clinical improvement in 38%.24
A limitation of this study is that it is retrospective with the restrictions that this involves in terms of data collection, of ophthalmological examination, and the characterisation of the types of uveitis, since not all the information was gathered systematically. Another limitation is the number of patients, which reduced when they were classified into subgroups, diminishing the statistical power of the study.
The use of immunosuppressive therapy and the new biological treatments with their potential side effects, advances in the early diagnosis of rheumatic diseases and the need for in-depth knowledge of ocular pathology, together with the need for precise measurement of ocular outcomes, evidence the importance of multidisciplinary clinics, where the knowledge of rheumatologists and ophthalmologists combine to enhance the follow-up and management of these patients.
In sum, almost half the patients with non-infectious uveitis required immunomodulators. The patients often required a change of combination of these drugs, and the percentage of patients who required a third change of medication ranged from 15% for the anterior uveitis cases to 42% for the intermediate cases.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that neither human nor animal testing has been carried out under this research.
Data confidentialityThe authors declare that they have complied with their work centre protocols for the publication of patient data.
Privacy rights and informed consentThe authors declare that no patients’ data appear in this article.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Millán-Longo C, Peiteado D, Schlincker A, Hidalgo V, Pieren A, Balsa A, et al. Uso de fármacos inmunomoduladores en una consulta de uveítis. Reumatol Clin. 2019;15:271–276.