Pulmonary involvement is a frequent and serious rheumatoid arthritis (RA) manifestation that affects 60%–80% of patients. CXCL10 is an inflammatory chemokine that regulates different biological responses, such as chemotaxis, angiogenesis, and inflammation.
AimThis study aimed to identify the role of CXCL10 as a peripheral blood marker of RA-ILD and its correlation with disease activity.
Patients and methodsThis cross-sectional study included 73 patients with RA (33 with ILD and 40 without ILD). Pulmonary function tests and high-resolution computed tomography were performed. Blood samples were taken for complete blood count and blood chemistry analysis, and human interferon-inducible protein 10 (IP-10/CXCL10) level. Statistical Package for the Social Sciences (version 22) was used for all statistical calculations.
ResultsThe serum CXCL10 level and patient age (r=.393, p=.024), disease duration (r=.756, p<0.001), erythrocyte sedimentation rate (r=.516, p=.002), C-reactive protein (r=.539, p=.001), and rheumatoid factor (r=.663, p<.001) revealed a significant positive correlation. Furthermore, the Modified Health Assessment Questionnaire (r=−.418, p=.015) revealed a significant negative correlation. Patients with RA-ILD show significantly higher CXCL10 than those without ILD (p<.001).
ConclusionCXCL10 is a useful RA disease activity biomarker and is an RA-ILD-sensitive biomarker, also CXCL10 is a significant predictor for development of RA-ILD.
La afección pulmonar es una manifestación frecuente y grave de la artritis reumatoide (AR) que afecta al 60-80% de los pacientes. CXCL10 es una quimiocina inflamatoria que regula diferentes respuestas biológicas, como la quimiotaxis, la angiogénesis y la inflamación.
PropósitoEste estudio tuvo como objetivo identificar el papel de CXCL10 como marcador en sangre periférica de RA-ILD y su correlación con la actividad de la enfermedad.
Pacientes y métodosEstudio transversal que incluyó a 73 pacientes con AR (33 con EPI y 40 sin EPI). Se realizaron pruebas de función pulmonar y tomografía computarizada de alta resolución. Se tomaron muestras de sangre para hemograma completo y análisis de química sanguínea y el nivel de proteína 10 inducible por interferón humano (IP-10/CXCL10). Se utilizó el paquete estadístico para las ciencias sociales (versión 22) para todos los cálculos estadísticos.
ResultadosEl nivel sérico de CXCL10 y la edad del paciente (r=0,393, p=0,024), la duración de la enfermedad (r=0,756, p<0,001), la velocidad de sedimentación globular (r=0,516, p=0,002), la proteína C reactiva (r=0,539, p=0,001) y el factor reumatoide (r=0,663, p<0,001) revelaron una correlación positiva significativa. Además, el Cuestionario de Evaluación de la Salud Modificado (r=−0,418, p=0,015) reveló una correlación negativa significativa. Los pacientes con RA-ILD muestran un CXCL10 significativamente mayor que aquellos sin ILD (p<0,001).
ConclusiónCXCL10 es un biomarcador útil de la actividad de la enfermedad de AR y es un biomarcador sensible a AR-ILD, también CXCL10 es un predictor significativo para el desarrollo de AR-ILD.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by joint inflammation, which causes symmetric stiffness, pain, swelling, motion limitation, and progressive cartilage and bone damage. This occurs due to inflammatory cell infiltration into the synovium, joints, and various body organs.1
A common and serious extra-articular manifestation of RA is pulmonary involvement in any lung compartment affecting 60%–80% of patients. Interstitial lung disease (ILD) is a particular type of lung involvement that is the leading cause of morbidity and mortality in ∼40% of patients with RA.2
RA interstitial lung disease (RA-ILD) pathogenesis involves a complex interaction of risk factors, including smoking history, male gender, and advanced age, and genetic factors, including HLA-DRB1 shared epitope and high titer of anticitrullinated peptide antibodies (ACPA). Data shows that dysregulated inflammatory cascades in RA-ILD can produce cytokines, chemokines, and growth factors that induce epithelial and endothelial cellular damage, angiogenesis, fibroblast proliferation, and, ultimately, lung fibrosis. ILDs have recently been on the spotlight because of severe lung damage and patients’ life quality.3–5
Interferon-gamma (IFN-γ) is an immunomodulatory cytokine with a significant role in RA pathogenesis.6 The chemokines are proteins with a chemotactic activity that target cells with chemokine receptors. They are categorized as C, CC, CXC, CX3C, and IFN-γ-inducible protein 10 (CXCL10, also called IP-10) chemokines. Monocytes, endothelial cells, neutrophils, mesenchymal cells, fibroblasts, and dendritic cells are among the cells that secrete CXCL10.7,8
CXCL10 is an inflammatory chemokine that regulates different biological responses, including inflammation, chemotaxis, and angiogenesis.6 CXCL10 is considered an ILD biomarker because of its pathogenesis involvement. B cells, type 1 T helper (Th1) cells, mast cells, dendritic cells, and fibroblasts all express CXCR3 in the synovium. CXCL10 controls Th1 cell migration from the blood to the synovium. Th1 type cell recruitment is diminished in patients with RA by inhibiting the reaction between CXCR3 and CXCL10, thereby proving the important role of CXCL10 in ILD pathogenesis.1
The current study aimed to identify the role of CXCL10 as a peripheral RA-ILD blood marker, the correlation of CXCL10 with disease activity, and to identify its association to RA-ILD.
Patients and methodsPatientsThis cross-sectional study included 73 patients (>18 years old) with RA (33 with ILD and 40 without ILD) fulfilling the 2010 American College of Rheumatology RA classification criteria.9,10 Convenient sampling was used to enroll patients in the study after the eligibility criteria assessment.
Full history was taken from patients, including age, gender, disease duration, present illness, drug utilization, age of disease onset, smoking status, duration of methotrexate intake, and family history. All patients were clinically evaluated for the disease activity score (DAS)-28.11
A Modified Health Assessment Questionnaire-Disability Index (MHAQ-DI) was used to assess the subjective physical function of patients with RA. Dressing, hygiene, rising, walking, reaching, gripping, eating, and usual activities are among the 20 items divided into eight categories.12,13
Patients suffering from other autoimmune disorders, such as systemic lupus erythematosus, dermatomyositis, and scleroderma, asthma, and chronic obstructive pulmonary disease, were excluded.
Pulmonary function testThe following measurements were taken: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and FEV1/FVC ratio. The restrictive ventilatory defect was defined on spirometric findings of FEV1/FVC ratio of <70% and FVC of <80%.14 Patients were functionally classified based on FEV1 representing the proportion of patients’ vital capacity which they can expire in the first second of forced expiration to full FVC. FEV1 classification includes severe (≤49), moderate (69–50), and mild (≤70).
Radiological investigation- 1.
Chest X-ray posteroanterior view
- 2.
High-resolution CT chest scan (HRCT)
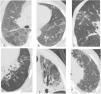
Early interstitial lung disease includes the following manifestations: septal thickening, reticulonodular opacities, ground glass opacities, mosaic appearance, emphysema, and cystic changes; while late manifestations include honey combing, tree in bud, crazy paving, consolidation, tractional bronchiectasis, and lung architecture distortion; Fig. 1. Study participants were divided into two groups based on the HRCT results, i.e., groups 1 (RA-ILD) and 2 (RA without ILD).
Typical MSCT findings of interstitial lung abnormalities. (A) Ground glass abnormalities, (B) reticular abnormalities with ground glass opacities, (C) tree in bud appearance, (D and E) traction bronchiectasis with reticulation and ground glass attenuation, and (F) honey combing demonstrated as clustered cystic air spaces.
A whole blood sample was divided into two parts: 2mL in an ethylenediaminetetraacetic acid tube for a complete blood picture and erythrocyte sedimentation rate and 3mL in a plain tube for serum separation. The serum is used for kidney and liver function tests, random blood glucose testing, C-reactive protein, and rheumatoid factor.
The last 2mL of blood was used for serum separation to determine human (IP-10/CXCL10) using Sun Red (201-12-0413).
The serum was coagulated at room temperature for 10–20min, followed by centrifugation for 20min at 2000–3000rpm to remove the supernatant. The specimen was stored at −20°C until its usage.
PrincipleThe kit employs a double-antibody sandwich enzyme-linked immunosorbent assay to determine the concentration of human IFN-inducible protein 10 (IP-10/CXCL10) in samples. The protein is incubated in a monoclonal antibody. The enzyme was well precoated with human IP-10 monoclonal antibody; then, IP-10/CXCL10 antibodies labeled with biotin were added and combined with Streptavidin-HRP to form an immune complex, which was repeatedly incubated and washed to remove the uncombined enzyme.
The study was approved by the Faculty of Medicine at Assiut University's Institutional Review Board, Egypt (no: 17101142), and was registered in clinical trials (NCT04356066).
Statistical methodsStatistical Package for the Social Sciences (version 22) was used for all statistical calculations. Data were statistically defined using mean±standard deviation (SD) or median (range) when not normally distributed, frequencies (number of cases), and relative frequencies (percentages) as appropriate. The Student's t-test was used for normally distributed data and the Mann–Whitney U test for non-normally distributed data to compare quantitative variables. The Kruskal–Wallis test was used to compare three quantitative variables. The chi-square2 test was used to compare categorical data, the exact test was used when the expected frequency is <5. Correlation between various variables was done using the Pearson correlation test. The best cutoff values for detecting ILD in patients with RA were determined using receiver operating characteristic curve (ROC) analysis. Odds ratio (OR) with 95% confidence interval (CI) and logistic regression was calculated for prediction of development of RA-ILD. p values of <0.05 are considered significant.
ResultsAmong the 73 enrolled patients with RA, evidence of interstitial lung abnormalities was found in 33 patients detected by HRCT. Patients suffering from RA-ILD were older with a longer disease duration than patients with RA without ILD (Table 1; p=0.001, and 0.002 respectively). Other demographic variables or environmental factors were comparable between both groups without significant difference between them regarding sex, smoking status, positive family history, and body mass index (p=0.622, 1, 0.744, and 0.379 respectively).
Baseline and clinical characteristics of RA patients with and without ILD (n=73).
| Variable name | ILD (n=33) | Without ILD (n=40) | p value |
|---|---|---|---|
| Age (years) | 52.52±10.21 | 43.70±10.35 | 0.001* |
| Median disease duration (years) | 10.00 (5.00–30.00) | 7.00 (1.00–20.00) | 0.002* |
| Sex, n (%) | 0.622 | ||
| Male | 1 (3.00) | 3 (7.50) | |
| Female | 32 (97.00) | 37 (92.50) | |
| Smoking status | 1 | ||
| No | 31 (93.94) | 37 (92.50) | |
| Yes | 2 (6.06) | 3 (7.50) | |
| Family history | 0.744 | ||
| Negative | 29 (87.88) | 33 (82.50) | |
| Positive | 4 (12.12) | 7 (17.50) | |
| BMI (kg/m2) | 26.56±5.71 | 27.78±5.97 | 0.379 |
| General manifestations, n (%) | |||
| Fever | 2 (6.06) | 1 (2.50) | 0.586 |
| Anorexia | 18 (54.55) | 16 (40.00) | 0.215 |
| Weight loss | 15 (45.45) | 12 (30.00) | 0.173 |
| Arthralgia | 28 (84.85) | 38 (95.00) | 0.233 |
| Arthritis | 11 (33.30) | 20 (50.00) | 0.152 |
| Morning stiffness | 8 (24.24) | 12 (30.00) | 0.583 |
| Subcut nodules | 2 (6.06) | 3 (7.50) | 1 |
| Deformity | 7 (21.21) | 15 (37.50) | 0.131 |
| Limitation of movement | 6 (18.18) | 13 (32.50) | 0.165 |
| Skin | 4 (12.12) | 1 (2.50) | 0.169 |
| Eye | 4 (12.12) | 1 (2.50) | 0.169 |
| CNS | 0 (0.00) | 0 (0.00) | – |
| Heart | 0 (0.00) | 0 (0.00) | – |
| Kidney | 0 (0.00) | 0 (0.00) | – |
| GIT | 0 (0.00) | 0 (0.00) | – |
| HTN | 1 (3.00) | 1 (2.50) | 1 |
| DM | 0 (0.00) | 1 (2.50) | 1 |
| Chest manifestations, n (%) | |||
| Cough | 32 (96.97) | 0 (0.00) | <0.001* |
| Pain | 33 (100.00) | 0 (0.00) | <0.001* |
| Expectoration | 33 (100.00) | 0 (0.00) | <0.001* |
| Dyspnea | 33 (100.00) | 0 (0.00) | <0.001* |
BMI: body mass index. Quantitative data are presented as mean±SD or median (range), qualitative data are presented as number (percentage).
Table 1 enumerated all the clinical features of the participants. General characteristics were comparable between both studied groups with no statistically significant difference (p>0.05) between them. Meanwhile, patients with RA-ILD suffer from more cough, pain, expectoration, and dyspnea compared to patients with RA without ILD (p<0.001).
Patients with ILD show higher disease activity as measured by the DAS28 score (p=0.007), more restricted PFT (p<0.001), more abnormal findings in HRCT (p<0.001), and lower life quality as measured by the MHAQ (p=0.033; Table 2).
DAS28, pulmonary function test, HRCT, MHAQ, and laboratory characteristics of RA patients with and without ILD (n=73).
| Variable name | ILD (n=33) | Without ILD (n=40) | p value |
|---|---|---|---|
| DAS28 categories | 0.007* | ||
| Low disease activity (2.6–3.2) | 9 (27.27) | 24 (60.00) | |
| Moderate disease activity (3.2–5.1) | 16 (48.48) | 14 (35.00) | |
| High disease activity (>5.1) | 8 (24.24) | 2 (5.00) | |
| Pulmonary function test | <0.001* | ||
| Normal | 0 (0.00) | 40 (100.00) | |
| 70 or more (mild) | 17 (51.52) | 0 (0.00) | |
| 69–50 (moderate) | 3 (9.09) | 0 (0.00) | |
| 49 or less (severe) | 13 (39.39) | 0 (0.00) | |
| HRCT | <0.001* | ||
| Normal | 0 (0.00) | 40 (100.00) | |
| Early ILD | 17 (51.52) | 0 (0.00) | |
| Late ILD | 16 (48.48) | 0 (0.00) | |
| MHAQ | 3.00 (0.00–7.00) | 6.00 (0.00–9.00) | 0.033* |
| CXCL10 (pg/mL) | 187.20 (102.50–2922.00) | 114.9 (20.40–1306.00) | <0.001* |
| ESR (mm/h) | 30.00 (7.00–80.00) | 27.00 (5.00–90.00) | 0.296 |
| C-reactive protein (mg/dl) | 15.00 (5.00–71.90) | 12.40 (1.20–52.20) | 0.004* |
| Rheumatoid factor (U/mL) | 40.00 (0.00–266.00) | 40.00 (0.00–265.00) | 0.466 |
| Serum urea (μmol/L) | 4.50 (2.20–27.00) | 4.40 (1.80–19.00) | 0.286 |
| Serum creatinine (μmol/L) | 54.90 (4.90–86.00) | 55.45 (4.80–99.00) | 0.729 |
| Serum uric acid (mg/dl) | 4.04±1.02 | 4.15±1.16 | 0.675 |
| Random blood glucose (mmol/L) | 5.60 (4.30–6.50) | 5.60 (4.50–10.00) | 0.566 |
| Total protein (g/L) | 71.00 (27.00–84.00) | 72.70 (64.00–87.00) | 0.474 |
| Albumin (g/L) | 41.00 (4.50–48.00) | 41.50 (34.00–44.50) | 0.460 |
| Total bilirubin (μmol/L) | 4.70 (0.60–11.00) | 2.80 (0.60–11.60) | 0.281 |
| Aspartate aminotransferase (U/L) | 23.00 (6.00–78.00) | 21.00 (4.00–55.00) | 0.163 |
| Alanine transaminase (U/L) | 23.00 (8.00–102.00) | 23.00 (8.00–51.00) | 0.602 |
| Alkaline phosphatase (U/L) | 80.00 (20.00–200.00) | 75.50 (16.00–179.00) | 0.447 |
| Urine | 0.455 | ||
| Normal | 28 (84.85) | 37 (92.50) | |
| Urate crystal | 5 (15.15) | 3 (7.50) | |
| Hemoglobin (g/dl) | 12.60 (8.20–15.40) | 12.25 (8.70–17.10) | 0.872 |
| Hematocrit (%) | 39.20 (28.00–47.30) | 37.35 (4.30–48.90) | 0.246 |
| Platelets (103/μl) | 278.76±68.48 | 284.55±69.16 | 0.722 |
| White blood cells (103/μl) | 6.90 (4.90–9.80) | 6.50 (4.10–9.60) | 0.381 |
HRCT: high resolution computed tomography; MHAQ: Modified Health Assessment Questionnaire; ESR: erythrocyte sedimentation rate. Quantitative data are presented as mean±SD or median (range), qualitative data are presented as number (percentage).
An insignificant difference was found between both studied groups regarding the type and dose of administered treatment, except for the number of patients with ILD who have received steroid treatment, which was statistically higher than patients without ILD (37 [74%] versus 7 [4%], p<0.001).
Patients with RA-ILD show significantly higher CXCL10, and C-reactive protein (CRP) than those without ILD (p<0.001, and 0.004, respectively; Table 2).
A significant positive association existed between the serum level of CXCL10 and the age of the studied patients (p=0.024), disease duration (years) (p<0.001), erythrocyte sedimentation rate (ESR) (p=0.002), CRP (p=0.001), and rheumatoid factor (RF) (p<0.001), with a negative significant correlation with MHAQ (p=0.015; Table 3).
The correlation between CXCL10 biomarker and demographic and clinical details of RA-ILD cases (n=33).
| Variable name | CXCL10 | |
|---|---|---|
| r | p | |
| Age (years) | 0.393 | 0.024* |
| Disease duration (years) | 0.756 | <0.001* |
| BMI | 0.232 | 0.194 |
| DAS28 score | 0.082 | 0.650 |
| MHAQ | −0.418 | 0.015* |
| ESR | 0.516 | 0.002* |
| CRP | 0.539 | 0.001* |
| RF | 0.663 | <0.001* |
BMI: body mass index; DAS28: disease activity score; MHAQ: Modified Health Assessment Questionnaire; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factor.
The means of CXCL10 were compared among different DAS categories using a one-way analysis of variance test, which showed a significant difference in the mean CXCL10 between groups with mild and high disease activities (p=0.046) but was insignificantly different between groups with mild and moderate disease activities (p=0.877). The mean CXCL10 in the moderate disease activity group was lower than in the high disease activity group (p=0.065; Table 4).
Comparison of the CXCL10 means according to DAS categories among RA-ILD patients (n=33).
| DAS28 categories | N | Median (range) | p-Valuea | p-Valueb | p-Valuec | p-Valued |
|---|---|---|---|---|---|---|
| Mild (≤3.2) | 9 | 145.90 (130.00–1460.00) | 0.037* | 0.877 | 0.046* | 0.065 |
| Moderate >3.2 and ≤5.1 | 16 | 240.60 (106.80–1510.00) | ||||
| High >5.1 | 8 | 973.10 (102.50–2922.00) |
DAS28: disease activity score: N: number.
ROC curve for ILD detection in patients with RA was done. CXCL10 has sensitivity and specificity for RA-ILD of 88% and 70%, respectively, at a cutoff of 128.15, with an area under the curve of 0.807 (0.72–0.894, p<0.001; Table 5 and Fig. 2).
The best cut off, sensitivity and specificity for RA-ILD detection by CXCL0 biomarker (n=73).
| Cut off | 95% CI | Sensitivity | Specificity | AUC | p-Value | |
|---|---|---|---|---|---|---|
| CXCL10 | 128.15 | 0.698–0.897 | 87.9% | 65.0% | 0.798 | <0.001* |
AUC: area under the curve; CI: confidence interval.
Univariate logistic regression analysis showed that age, disease duration, CXCL10, and CRP variables were significantly associated with RA-ILD. This finding was confirmed on multivariate logistic regression analysis which shows that age, and CXCL10 variables are still significantly associated with RA-ILD. CXCL10 was the most associated factor among them; were patients with CXCL10 ≥128 were about 10 times more likely to developed RA-ILD as compared to patients with CXCL10 <128 (OR=9.566, 95% CI 2.492–36.716, p<0.001; Table 6).
Univariate and multivariate logistic regression analysis for prediction of RA-ILD by different laboratory data (n=73).
| Variables | n | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Age | 73 | 1.084 | 1.032–1.138 | 0.001* | 1.065 | 1.001–1.134 | 0.045* |
| Disease duration | 73 | 1.160 | 1.048–1.285 | 0.004* | 1.048 | 0.921–1.193 | 0.474 |
| CXCL10 | |||||||
| <128 | 30 | Ref | Ref | ||||
| ≥128 | 43 | 13.464 | 3.932–46.103 | <0.001* | 9.566 | 2.492–36.716 | 0.001* |
| CRP | 73 | 1.073 | 1.019–1.129 | 0.007* | 1.033 | 0.967–1.103 | 0.339 |
ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; CI: confidence interval; OR: odds ratio.
One of the mortality causes in patients with RA is interstitial lung disease, highlighting the need for biomarkers to identify patients at risk of developing ILD.15 This study aimed to evaluate the serum CXCL10 level as a biomarker for RA-ILD by including 73 patients with RA, of whom 33 had evidence of lung abnormalities detected by HRCT. In RA-ILD, dysregulated inflammatory cascades can produce cytokines, chemokines, and growth factors that cause epithelial and endothelial cellular damage, angiogenesis, fibroblast proliferation, and, eventually, lung fibrosis. CXCL10 is an inflammatory chemokine that controls a variety of biological responses such as inflammation, chemotaxis, and angiogenesis. CXCR3 is expressed in the synovium by B cells, type 1 T helper (Th1) cells, mast cells, dendritic cells, and fibroblasts. CXCL10 regulates the migration of Th1 cells from the blood to the synovium. Th1 cell recruitment is reduced in RA patients by inhibiting the reaction between CXCR3 and CXCL10, demonstrating the importance of CXCL10 in ILD pathogenesis. Individuals with RA-ILD were found to be older and had longer disease duration than patients with RA without ILD due to lung fibrosis by comparing the baseline demographic data among both studied groups. In line with the findings of the current study, Chen et al., Restrepo et al., and Salaffi et al.5,16,17 discovered that patients with RA-ILD were older and had a longer disease duration than those with RA without ILD.
The provided information is not exhaustive regarding gender and ILD; some researchers discovered a positive association between male gender and RA-ILD16,18,19; however, others did not find such a correlation as the current study found.20,21 This could be due to the higher prevalence of RA among female patients, which will also explain the absence of the difference between smoking status and ILD development among the patients in the current study with RA due to the lower smoking rate among Egyptian females.
Regarding the clinical presentation of the studied participants, the current study revealed that patients with RA-ILD were suffering from higher cough, pain, expectoration, dyspnea, and more restricted PFT (p<0.001) as compared to those with RA without ILD (p<0.001 for all), which was in line with Chen et al. and Spagnolo et al.2,5
The present study used DAS28-ESR and revealed that patients with RA-ILD have higher significant disease activity than those without ILD (p=0.007). Congruent with the current study, Chen et al. and Restrepo et al.5,16 observed that patients with RA-ILD have higher DAS28 scores than those without ILD. However, Kass et al. and Salaffi et al.4,17 discovered insignificant differences in the measurement of DAS28-ESR between both groups because the authors reported differences in the methotrexate usage and tumor necrosis factor inhibitors between those groups, with both drugs usage being higher in the RA without ILD subgroups than with ILD (p=0.05).
The current research regarding MHAQ revealed that patients with RA-ILD suffered from lower life quality as compared to those without ILD (p=0.033). This is mainly due to cough, pain, expectoration, and dyspnea, which were more common among patients with RA-ILD. The current study supported the studies of Natalini et al. and Fadda et al.22,23 who reported that patients with RA-ILD suffered from lower life quality compared to those without ILD as assessed by MHAQ (p=0.009).
Patients with RA-ILD were found to show significantly higher CRP than those without ILD (p=0.004). Several studies were in line with the current study in comparing different laboratory findings between both studied groups.16,19,24–27 In contrast to the current study, Fadda et al.23 and Salaffi et al.17 found no statistically significant differences in CRP levels between both studied groups. This conflict between the previously mentioned studies could be explained by the degree of RA activity during the assessment of this inflammatory serological marker.
According to the current findings, patients with RA-ILD have higher significant CXCL10 levels than those without ILD (p<0.001). In line with the findings of the current study, Chen et al.5 discovered that serum CXCL10 levels were significantly higher in patients with RA-ILD. Kameda et al.28 recently reported that serum CXCL10 levels and bronchoalveolar lavage fluid were significantly higher in patients suffering from interstitial pneumonia with autoimmune features or collagen vascular diseases associated with ILD than in patients with IPF.
The mean CXCL10 was discovered to be highly significant among patients with RA suffering from high disease activity when compared with the mean CXCL10 across different DAS28-ESR categories. Additionally, a positive significant correlation was discovered between CXCL10 and age, disease duration, CRP, ESR, and RF, as well as a negative significant correlation with MHAQ. Furthermore, the CXCL10 and disease activity correlation provides stronger proof that this serum protein can be utilized as an efficient biomarker for the pathology of specific organs in systemic inflammatory disorder. Whereas the comparatively high CXCL10 level in patients with RA-ILD with higher disease activity reinforces that extra-articular complication is caused by a conjunction of immunity dysregulation and excessive tissue remodeling. Supporting the current findings, Chen et al. and Kuan et al.5,29 stated that serum and CXCL10 concentrations may be utilized as a disease activity biomarker in patients with RA.
The role of the CXCL10 biomarker was previously examined in RA pathogenesis; however, its level has never been studied to reflect disease activity in patients with RA, except in two previously mentioned studies by Kuan et al. and Chen et al.5,29 Hence, the findings of the current study cannot be compared, and larger sampled studies are required to confirm the findings.
Multivariate logistic regression analysis which shows that age, and CXCL10 and CRP variables are still significant predictors for RA-ILD. The most significant predictor among them was CXCL10 (p=0.001). Mori et al.20 stated that logistic regression analysis indicated a strong association of ILD with age, smoking and titers of RF, also Salaffi et al.17 when studied multivariate regression analysis, they highlighted the correlation between age, ACPA titer, age at RA onset, smoking, and ILD. Additionally, Muhsin et al.30 cleared that on regression CXCL10 was significant predictor for RA. Kotrych et al.31 stated that regression analysis with multiple variables taking into account patient gender and age, disease duration, and the CXCL10 GG genotype, this genotype was found to be the independent factor associated with an increased risk of developing extra-articular manifestations.
Limitation and recommendationSimilar studies need to be conducted on a larger number of patients with RA-ILD, and further studies should estimate ACPA and its correlation with CXCL10 in RA-ILD.
ConclusionCXCL10 is a good biomarker for RA disease activity and is a sensitive biomarker for RA-ILD. Additionally, CXCL10 is a significant predictor for development of RA-ILD.
Conflict of interestsThe authors declare they have no conflict of interest.