Glomerulonephritis is a heterogenous group of diseases which is diagnosed mainly by renal biopsy. This study aims to assess nailfold capillaroscopic changes in patients with glomerular diseases.
Patients and methodsThis study was conducted on 50 patients with glomerular disease confirmed by renal biopsy and 50 age and sex matched healthy controls. Clinical, laboratory evaluation and nailfold capillaroscopic examination were done for all participants.
ResultsLupus nephritis was the most common pathological type among glomerulonephritis group [17 (34%)], followed by focal segmental glomerulosclerosis [9 (18%)]. Tortuous capillaries were significantly higher in patients with primary glomerular diseases compared to healthy persons (92.9% vs 58%, P<0.001). The diameter was significantly lower in patients with primary glomerular diseases compared to healthy control group (15.6μm vs 18.9μm, P=0.001). Subpapillary venous plexus (28.6% vs 6%, P=0.02) and capillary microhemorrhage (32% vs 0%, P<0.001) were significantly more frequent in patients with primary glomerular diseases compared to the control group. Patients with lupus nephritis had higher capillary diameter (19.5 vs 15.6μm, P=0.02), width (55.6 vs 44μm, P=0.003) and microhemorrhage (64.7% vs 32%, P=0.03) compared to patients with primary glomerulonephritis. There was a statistically significant difference as regard diameter (P=0.005) and length (P=0.02) between different classes of lupus nephritis.
ConclusionMore tortious capillaries and lower capillary diameter were found in patients with primary glomerular disease compared to healthy persons. Lupus nephritis patients had more dilated capillaries and more capillary microhemorrhage compared to primary glomerulonephritis patients.
Las glomerulonefritis son un grupo heterogéneo de enfermedades que se diagnostican principalmente mediante biopsia renal. Este estudio tiene como objetivo evaluar los cambios capilaroscópicos del pliegue ungueal en pacientes con enfermedades glomerulares.
Pacientes y métodosEste estudio se realizó en 50 pacientes con enfermedad glomerular confirmada mediante biopsia renal y 50 controles sanos emparejados por edad y sexo. A todos los participantes se les realizó una evaluación clínica, de laboratorio y un examen capilaroscópico del lecho ungueal.
ResultadosLa nefritis lúpica fue el tipo patológico más común entre el grupo de glomerulonefritis (17 [34%]), seguida de la glomeruloesclerosis focal y segmentaria (9 [18%]). Los capilares tortuosos fueron significativamente mayores en pacientes con enfermedades glomerulares primarias en comparación con personas sanas (92,9%, 58%, p <0,001). El diámetro fue significativamente menor en pacientes con enfermedades glomerulares primarias en comparación con el grupo de control sano (15,6μm vs. 18,9μm, p=0,001). El plexo venoso subpapilar (28,6% vs. 6%; p=0,02) y la microhemorragia capilar (32% vs. 0, p <0,001) fueron significativamente más frecuentes en pacientes con enfermedades glomerulares primarias en comparación con el grupo de control. Los pacientes con nefritis lúpica tuvieron mayor diámetro capilar (19,5 vs. 15,6μm, p=0,02), ancho (55.6 vs. 44μm, p=0,003) y microhemorragia (64,7% vs. 32%, p=0,03) en comparación con los pacientes con glomerulonefritis primaria.
ConclusiónSe encontraron capilares más tortuosos y menor diámetro capilar en pacientes con enfermedad glomerular primaria en comparación con personas sanas. Los pacientes con nefritis lúpica tenían capilares más dilatados y más microhemorragia capilar en comparación con los pacientes con glomerulonefritis primaria.
Glomerulonephritis (GN) is a heterogenous group of diseases characterized by increased glomerular cellularity due to proliferation of glomerular cells and/or leukocyte infiltration which may lead to glomerular damage. If GN is not properly treated may progress to chronic kidney disease and end stage renal disease (ESRD).1 GN is the most common cause of end stage renal disease in young people. The clinical presentation of GN is variable, ranging from asymptomatic proteinuria to rapidly progressive GN with hypertension, oliguria and uremia. The classic presentation of acute GN includes edema, hypertension hematuria and sometimes oliguria. Patients may also present with nephrotic syndrome and generalized edema.2
Renal biopsy is the gold standard for diagnosis of GN as it can differentiate it from other causes of kidney disease, can identify the histopathological type and differentiate active reversible lesions from chronic irreversible lesions. Renal biopsy should be evaluated by light microscopy, immunohistology and often by electron microscopy.2 GN can be classified according to the pathogenesis into immune-complex GN, pauci-immune GN, anti-glomerular basement membrane antibody (anti-GBM) GN, monoclonal Ig GN, and C3 glomerulopathy.3
Nailfold capillaroscopy (NFC) is an easy, noninvasive method for examination of nailfold capillaries. Normal nailfold capillaroscopy appear as homogenous parallel hairpin shaped capillaries.4 NFC is strongly recommended for patients with Raynaud's phenomenon and is commonly used by rheumatologists in clinical practice and in research studies. Presence of giant capillaries, microhemorrhage, avascular areas and neo angiogenesis is helpful for diagnosis of scleroderma spectrum disorders.5
In systemic sclerosis, NFC is included in the diagnostic criteria, can be used for early diagnosis and can identify the activity phase (either early, active or late pattern). In other connective tissue diseases as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), NFC changes are nonspecific but may provide support to the diagnosis. The utility of NFC in non-rheumatic diseases is not well studied.6 NFC may be helpful in assessment of microcirculation and endothelial dysfunction. Capillaroscopic changes in the form of decrease in capillary density was reported in association with chronic kidney disease (CKD).7 Also more decrease in capillary density was found in CKD patients with diabetes mellitus (DM) than CKD patients without DM.8 Additionally, NFC changes was associated with disease severity in ANCA (antineutrophil cytoplasmic antibody) associated vasculitis.9 However, no previous studies evaluated the capillaroscopic changes in patients with glomerular diseases.
Patients and methodsThis study aims to assess NFC changes in patients with glomerular diseases. This is a comparative cross-sectional study which was conducted on 50 patients with glomerular disease who attended and/or referred to the nephrology or Rheumatology and Immunology units and outpatient clinics, Mansoura university hospital beside 50 healthy controls. The study was conducted from March 2022 to March 2023. This study was approved by Institutional Review Board of Mansoura university (IRB code number: MS.21.12.1777). The study was explained to all the participants and an informed consent was taken.
Patients older than 16 years who were diagnosed to have glomerular disease based on renal biopsy were included in this study. Patients less than 16 years and patients who did not take renal biopsy due to any cause were excluded from this study. Lupus nephritis patients with overlap with other connective tissue diseases were excluded from this study. Participants were divided into two groups. Group 1 included 50 patients diagnosed with glomerular disease, group 2 which included 50 age and gender matched healthy persons.
All participants were subjected to full history taking and examination including age, smoking and chronic illness. Investigations were done to the patients including complete blood count, serum creatinine, urine analysis, 24-h urinary protein, ESR (erythrocyte sedimentation rate) and CRP (C reactive protein). The result of renal biopsy was revised and documented in all patients.
Capillaroscopic examinationCapillaroscopic examination was done for all participants in this study using videocapillaroscope by Dino lite with 200× magnification. Participants were seated in a temperature-controlled (23–26°C) room for 15–20min for relaxation and acclimatization.10 Capillaroscopic examination was done for eight fingers of both hands (excluding the thumbs) in each participant. Fingers with thick nailfolds and gangrenous fingers were not analyzed. A drop of vegetable oil was applied to the nailfold of each finger before examination to maximize the amount of light and improve the image quality.11 Care was taken not to exert too much pressure on the nail surface. Three adjacent fields were examined for nailfold of each finger by one Rheumatologist trained in this technique and was blinded to the clinical data of the patients.12 The images were analyzed using the software Dino Capture 2 version 1.5.47.B, in which measurements of the capillaries were made and the characteristics of the capillary microarchitecture were carefully visualized.
The parameters measured include capillary density, capillary width, capillary diameter and capillary length. Capillary density means the number of capillaries in a 1mm length of the distal row of each finger. Capillary width is the width of a capillary loop at its widest section. The diameter of capillaries at its widest sections was also measured.13 The average was calculated for each of the observed parameters in 24 images.
Capillary shape was evaluated, and regular hairpin shaped capillaries was considered normal.11 Tortious capillaries are capillaries which intersect like number eight with convex apex. Abnormal capillary shapes include branched, bushy and bizarre capillaries.14 If more than 50% of the existing capillaries were evaluated as being affected, they were scored as “present”. If less than 50% of capillaries were found to be affected, they were scored as “none”. Patients were also evaluated for the presence of capillary microhemorrhage and subpapillary venous plexus into either present or absent. Capillary microhemorrhages mean presence of 2 or more punctate bleeds or hemosiderin deposits around a single capillary in at least 2 fingers. The vascular network at the base of a finger nailfold into which capillaries drain is called the subpapillary plexus.13
Statistical analysisThe results were collected, sorted & tabulated using computer based spread sheet program. The collected data were analyzed by SPSS. Numerical data were presented as means±standard deviation when they were parametric and median when they were non-parametric. Non numerical data were presented as frequencies & percentage. Data were tested for normality via Kolmogorov–Smirnov test. In the normally distributed variables, T test and one way ANOVA test were used; while in non-normally distributed variables, Mann Whitney and Kruskal–Wallis tests were used. Association between 2 continuous variables was done by Pearson's correlation, whereas non-parametric associations were done by spearman's rank correlation. Regression analysis models will be used to define independent predictors. P value≤0.05 was considered statistically significant.
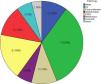
ResultsLupus nephritis was found in 17 patients and primary glomerular diseases were found in 28 patients. The remaining patients had other glomerular diseases: 1 patient with diabetic nephropathy, 1 patient with renal amyloidosis and 3 patients with crescentic glomerulonephritis secondary to vasculitis. Lupus nephritis was the most common pathological type [17 (34%)], followed by focal segmental glomerulosclerosis (FSGS) [9 (18%)] as demonstrated in Fig. 1.
Patients with primary glomerular diseases had more common tortious capillaries as in Fig. 2 (92.9%) however healthy persons had more common hairpin capillaries as in Fig. 2 (58%) (P<0.001). Capillary diameter was significantly lower in patients with primary glomerular diseases compared to healthy control (15.6 vs 18.9μm, P=0.001). Patients with primary glomerular diseases had more frequent capillary hemorrhage (32% vs 0, P<0.001) and subpapillary venous plexus as in Fig. 3 (28.6% vs 6%, P=0.02) compared to the healthy control group. There was no significant difference between patients with primary glomerular diseases and healthy control group as regard capillary density (6.9 vs 7.2capillary/mm, P=0.3), architecture, width (43.9 vs 42.2μm, P=1), and length (265.9 vs 268.8μm, P=0.7) as in Table 1.
Difference in capillaroscopic characteristics between patients with primary glomerular diseases and the healthy control group.
| Primary glomerulonephritis | Control group | P value | |
|---|---|---|---|
| N=28 | N=50 | ||
| Capillary density (capillary/mm)Mean±SD | 6.9±1 | 7.2±1.1 | 0.3 |
| Capillary shape | |||
| Hair pin n (%) | 0 | 29 (58%) | <0.001* |
| Tortious n (%) | 26 (92.9%) | 21 (42%) | |
| Abnormal n (%) | 2 (7.1%) | 0 | |
| Capillary architecture | |||
| Normal n (%) | 27 (96.4%) | 50 (100) | 0.4 |
| Disturbed n (%) | 1 (3.6%) | 0 | |
| Diameter (μm)Mean±SD | 15.6±3.5 | 18.9±3.9 | 0.001* |
| Length (μm)Mean±SD | 265.9±113.6 | 268.8±89.3 | 0.7 |
| Width (μm)Mean±SD | 43.9±11 | 43.2±11.98 | 1 |
| Subpapillary venous plexus n(%) | 8 (28.6%) | 3 (6.0%) | 0.02* |
| Capillary hemorrhage n(%) | 9 (32%) | 0 | <0.001* |
SD: standard deviation.
There were no statistically significant differences between patients with primary glomerulonephritis and patients with lupus nephritis as regards demographic, clinical and laboratory data except for 24-hour urinary protein which was significantly higher in patients with primary glomerulonephritis (5 vs 2g/24h, P=0.005) as demonstrated in Table 2. Patients with lupus nephritis had higher capillary diameter as in Fig. 4 (19.5 vs 15.6μm, P=0.02), width as in Fig. 4 (55.6 vs 44μm, P=0.003) and microhemorrhage as in Fig. 5 (64.7% vs 32%, P=0.03) compared to patients with primary glomerulonephritis. Otherwise, there was no statistically significant difference between patients with primary glomerulonephritis and patients with lupus nephritis as regards other capillaroscopic parameters as illustrated in Table 3.
Difference in sociodemographic, clinical and laboratory characteristics between patients with primary glomerulonephritis and patients with lupus nephritis.
| Primary glomerulonephritis | Lupus nephritis | P value | |
|---|---|---|---|
| N=28 | N=17 | ||
| Age/years | |||
| Median (IQR) | 36 (21–43.75) | 36 (24–40) | 0.9 |
| Sex | |||
| Male n (%) | 11 (39.3%) | 3 (17.6%) | 0.2 |
| Female n (%) | 17 (60.7%) | 14 (82.4%) | |
| Hypertension n (%) | 14 (50%) | 11 (64.7%) | 0.3 |
| Diabetes n (%) | 4 (14.3%) | 2 (11.8%) | 0.6 |
| Smoking n (%) | 2 (7.1%) | 2 (11.8%) | 0.5 |
| 24h urinary protein (g/day) | |||
| Median (IQR) | 5 (1.53–6.85) | 2 (.055–2.5) | 0.005* |
| Serum creatinine (mg/dl) | |||
| Median (IQR) | 1.3 (1–2) | 1 (0.9–1.8) | 0.5 |
| Hemoglobin (g/dl) | |||
| Mean±SD | 10.5±2.1 | 10.3±2.2 | 0.6 |
| CRP | |||
| Positive n (%) | 12 (42.6%) | 4 (23.5%) | 0.2 |
SD: standard deviation, IQR: interquartile range, CRP: C reactive protein.
Difference in capillaroscopic characters between patients with primary glomerulonephritis and patients with lupus nephritis.
| Primary glomerulonephritis | Lupus nephritis | P value | |
|---|---|---|---|
| n=28 | n=17 | ||
| Capillary density (capillary/mm)Mean±SD | 6.9±1 | 6.8±0.7 | 0.9 |
| Capillary shape | |||
| Hair pin n (%) | 0 | 0 | 0.4 |
| Tortious n (%) | 26 (92.9%) | 17 (100%) | |
| Abnormal n (%) | 2 (7.1%) | 0 | |
| Capillary architecture | |||
| Normal n (%) | 27 (96.4%) | 17 (100%) | 0.6 |
| Disturbed n (%) | 1 (3.6%) | 0 | |
| Diameter (μm)Mean±SD | 15.6±3.5 | 19.5±5.7 | 0.02* |
| Length (μm)Mean±SD | 230 (165–354) | 218 (156–318) | 0.6 |
| Width (μm)Mean±SD | 43.99±11 | 55.6±13.6 | 0.003* |
| Subpapillary venous plexus n (%) | 8 (28.6%) | 3 (17.6%) | 0.3 |
| Capillary hemorrhage n (%) | 9 (32%) | 11 (64.7%) | 0.03* |
SD: standard deviation, IQR: interquartile range.
There were no statistically significant differences in capillaroscopy between patients with different types of primary glomerulonephritis as illustrated in Table 4. There was a statistically significant difference as regard diameter (P=0.005) and length (P=0.02) between different classes of lupus nephritis. Post hoc analysis revealed that capillary diameter was significantly higher in class V lupus nephritis compared to both class III and class IV (P<0.001, P=0.004 respectively). Capillary length in class III was significantly lower than patients with class IV and class V lupus nephritis (P=0.03, P=0.02 respectively). While no significant difference was detected as regard the remaining capillaroscopic parameters as demonstrated in Table 5.
Difference in capillaroscopy between patients with different types of primary glomerulonephritis.
| MPGN | Focal proliferative glomerulonephritis | Minimal change disease | FSGS | Membranous nephropathy | P value | |
|---|---|---|---|---|---|---|
| N=5 | N=5 | N=3 | N=9 | N=6 | ||
| Capillary density (capillary/mm)Mean±SD | 7±1.3 | 6.5±1 | 8±1 | 6.6±0.7 | 7±0.8 | 0.3 |
| Capillary shape | ||||||
| Hair pin n (%) | 0 | 4 (80%) | 0 | 0 | 0 | |
| Tortious n (%) | 5(100%) | 1 (20%) | 3 (100%) | 8 (89%) | 6 (100%) | 0.7 |
| Abnormal n (%) | 0 | 0 | 0 | 1 (11%) | 0 | |
| Capillary architecture | ||||||
| Normal n (%) | 4 (80%) | 5 (100%) | 3 (100%) | 9(100%) | 6 (100%) | 0.3 |
| Disturbed n (%) | 1 (20%) | 0 | 0 | 0 | 0 | |
| Diameter (μm)Mean±SD | 13.7±2.3 | 15±4.5 | 12.9±3.3 | 17.3±3.2 | 16.6±3.3 | 0.2 |
| Length (μm)Mean±SD | 239±65 | 209±50 | 218±109 | 315±158 | 285±97 | 0.7 |
| Width (μm)Mean±SD | 37.7±7.5 | 48.6±17.8 | 38.9±4.6 | 44±5.5 | 47.3±14.5 | 0.8 |
| Subpapillary venous plexus n (%) | 3 (60%) | 1 (20%) | 0 | 0 | 1 (16.7%) | 0.4 |
| Capillary hemorrhage n (%) | 2 (40%) | 2 (40%) | 1 (33.3%) | 3 (33.3%) | 1 (16.7%) | 0.9 |
SD: standard deviation, IQR: interquartile range, MPGN: membranoproliferative glomerulonephritis, FSGS: focal segmental glomerulosclerosis.
Difference in capillaroscopy between patients with different classes of lupus nephritis.
| Class III | Class IV | Class V | P value | |
|---|---|---|---|---|
| N=5 | N=8 | N=3 | ||
| Capillary density (capillary/mm)Mean±SD | 7.1±0.36 | 6.7±0.7 | 6.7±1.2 | 0.14 |
| Capillary shape | 1 | |||
| Hair pin n (%) | 0 | 0 | 0 | |
| Tortious n (%) | 5 (100%) | 8 (100%) | 3 (100%) | |
| Abnormal n (%) | 0 | 0 | 0 | |
| Capillary architecture | 1 | |||
| Normal n (%) | 5 (100%) | 8 (100%) | 3 (100%) | |
| Disturbed n (%) | 0 | 0 | 0 | |
| Diameter (μm)Mean±SD | 15.5±1.2 | 19.4±3.4 | 28.2±6.7 | 0.005*P1=0.09P2=0.004*P3<0.001* |
| Length (μm)Mean±SD | 162±34 | 268±101 | 322±41 | 0.02*P1=0.03*P2=0.3P3=0.02* |
| Width (μm)Mean±SD | 44.8±5.6 | 57.6±10.6 | 70.3±19.4 | 0.08 |
| Subpapillary venous plexus n (%) | 2 (40%) | 8 (100%) | 1 (33.3%) | 0.2 |
| Capillary hemorrhage n (%) | 3 (60%) | 4 (50%) | 3 (100%) | 0.3 |
SD: standard deviation, IQR: interquartile range. P1 difference between class III and class IV, P2 difference between class IV and class V, P3 difference between class III and class IV.
There were no statistically significant correlations between proteinuria and all capillaroscopic parameters except for capillary hemorrhage which had a significant negative correlation with proteinuria (R=−0.4, P=0.006). In addition, there was a significant positive correlation between proteinuria and serum creatinine (R=0.4, P=0.004) as in Table 6. Binary logistic regression was done and revealed that capillary diameter was a predictor for the presence of primary glomerulonephritis among all studied participants (P=0.002). However, diameter, width and capillary hemorrhage were not significant predictors for the presence of lupus nephritis in patients with glomerular diseases.
Correlation between proteinuria, capillaroscopic parameters and serum creatinine.
| Patients with glomerular diseases | ||
|---|---|---|
| Character | Rho | P |
| Capillary density | 0.02 | 0.9 |
| Capillary shape | 0.02 | 0.9 |
| Capillary architecture | 0.05 | 0.7 |
| Capillary diameter | 0.09 | 0.5 |
| Capillary length | 0.03 | 0.9 |
| Capillary width | −0.3 | 0.06 |
| Capillary hemorrhage | −0.4 | 0.006* |
| Subpapillary venous plexus | 0.08 | 0.6 |
| Serum creatinine | 0.4 | 0.004* |
Glomerular diseases are relatively rare kidney diseases which can lead to CKD and ESRD. Kidney biopsy is the diagnostic procedure of choice.15 This study was conducted on 50 patients with glomerular diseases who were compared to 50 healthy control participants. Nailfold capillaroscopic examination was done for all participants. The commonest GN type was lupus nephritis followed by FSGS and membranous nephropathy. Capillaroscopic characteristics showed significant differences between primary GN patients and control group as regard capillary shape, diameter, subpapillary venous plexus and capillary hemorrhage. There was statistically significant difference between lupus nephritis patients and patients with primary glomerulonephritis as regard diameter, width and capillary hemorrhage. There was a significant negative correlation between proteinuria and capillary hemorrhage.
In the present study, lupus nephritis was the commonest cause for GN. In agreement with this, recent Egyptian study on 140 Egyptian GN patients reported that lupus nephritis was the commonest cause for secondary GN and FSGS was the commonest primary cause for GN (21.4%).16 Similarly, other studies reported that lupus nephritis was the most common cause of glomerulonephritis.17–19 The most common primary GN in the current study was FSGS followed by membranous nephropathy. Similar to this, other studies reported that FSGS was the most frequent primary GN.19,20 Membranous nephropathy was the most common primary GN in other studies.18,21 While, in a recent Japanese study, minimal change disease was the commonest GN representing 56.7% followed by membranous nephropathy then FSGS.22 These different results may be related to difference in the ethnicity between patients in different studies.
In the present study, capillaroscopic characteristics between primary GN patients and control group were compared. No previous studies evaluated the capillaroscopic characteristics of glomerulonephritis patients. However, some studies evaluated these characteristics among chronic kidney disease, diabetic nephropathy and autoimmune rheumatic diseases.7,23,24
As regards capillary density and architecture there was no significant difference between primary GN and control groups in the current study. Similarly, there was no statistically significant differences between hemodialysis patients, renal transplant recipients and control groups as regard capillary density and architecture.25 On contrary, Thang et al. found significantly lower capillary density in ESRD patients compared to healthy control.26 In another study conducted on 96 CKD patients, capillary density was also lower in CKD patients compared to healthy control and capillary density was found to decrease with CKD progression.7 This may explain the absence of difference between GN patients and healthy control group in this study as most of our patients have normal kidney function and the mean serum creatinine was 1.1mg/dl.
In the present study there was statistically significant difference between primary GN and control group as regard capillary shape with higher percent of tortious capillaries among primary GN group. This was in concordance with the results of previous study conducted on 144 patients with diabetic nephropathy and reported that patients with proteinuria had higher incidence of tortious capillaries than normoalbuminuric patients and healthy control group.23 In other two studies, tortious capillaries had higher prevalence in patients with diabetic nephropathy and diabetic microangiopathy than healthy control.27,28
The current study showed significantly lower capillary diameter in patients with primary GN compared to healthy control group. This result was different from previous two studies which demonstrated no difference in capillary diameter between patients with renal disease and diabetic nephropathy on one side and healthy control.25,29 In the current study, subpapillary venous plexus was significantly more common in GN patients than healthy control group. On the other hand, Bakirci et al. did not find statistically significant difference between diabetic nephropathy group and non-diabetic nephropathy group as regard percent of subpapillary venous plexus.29
Capillary hemorrhage was more frequent in patients with primary GN than healthy control group and this was similar to the results of previous study which demonstrated significant difference in capillary hemorrhage between diabetic patients with proteinuria and those without proteinuria.23 However other studies failed to demonstrate significant difference between patients with renal disease and diabetic nephropathy in one hand and healthy control group in the other hand.25,29,27 This difference can be explained by the difference in the cause of renal disease between the studies.
Comparison between primary glomerulonephritis and lupus nephritis as regard capillaroscopic changes showed that lupus nephritis patients had more aggressive changes than primary glomerulonephritis especially in capillary diameter, width, and hemorrhage. This was in agreement with what was reported by Raeeskarami et al. that SLE patients had lower capillary density than healthy control.24 Other studies reported high frequency of tortious capillaries among SLE patients.30–32 Also, capillary diameter was significantly higher in SLE patients than healthy control in multiple studies.33 However, Fatemi et al. reported no significant difference in capillary density between SLE patients and healthy control group.24,34
There was statistically significant difference in capillary diameter and length between different classes of lupus nephritis in this study. Another study showed that capillary abnormalities were very common in patients with SLE and some changes may be associated with disease activity as elongated capillary loops which were seen more often in patients with renal involvement.35 Similarly, Hamza et al. found that progression of NFC score had been directly related to lupus activity and internal organ involvement especially nephritis.30 Another study showed that NFC abnormalities were correlated with disease activity in Egyptian SLE patients.36
The significant difference between lupus nephritis and other GN as regard capillaroscopic changes could be explained by the add on effect of SLE disease itself. As reported in multiple studies, SLE was associated with significant capillaroscopic changes as low capillary density, increased capillary tortuosity and increased capillary diameter.30,34,31 The capillaroscopic changes in SLE patients were attributed to increased VEGF (vascular endothelial growth factor) levels and interleukin-18 levels in SLE causing endothelial hyperplasia and damage leading to microcirculatory changes.31
The present study showed significant negative correlation between proteinuria and capillary hemorrhage. In agreement with this, Cutolo et al. reported statistically significant inverse correlation between Capillaroscopic changes and degree of proteinuria in SLE patients.37 Similarly, another study showed that capillaroscopic changes were significantly correlated with markers of SLE activity and 24-h urinary protein.38 In this study, there were no statistically significant correlations between serum creatinine and any of capillaroscopic changes in glomerular disease patients. This was similar to the results of previous studies which failed to demonstrate statistically significant correlation between serum creatinine and capillaroscopic findings in SLE patients.24,35,39
The main advantage of this study is that it is the first study to evaluate the capillaroscopic findings in GN patients. Another advantage is that the study compared capillaroscopic changes between primary GN and healthy control and between primary GN and lupus nephritis. The main limitation of this study is the small number of patients in each group of primary GN. Another limitation is that the effect of treatment and improvement of proteinuria was not evaluated in this study. Further studies are required on larger groups of patients with different types of primary GN to demonstrate if there is a specific capillaroscopic pattern in each type. Also, further longitudinal studies are required to study the effect of improvement of proteinuria on capillaroscopy.
ConclusionCapillaroscopy may be helpful in the diagnosis of type and cause of glomerular disease. There were significant differences between primary GN patients and control group as regard capillary shape, diameter, subpapillary venous plexus and capillary hemorrhage. There were statistically significant differences between lupus nephritis patients and patients with primary GN as regard diameter, width and capillary hemorrhage. However, there was no significant difference in capillaroscopic changes between different types of primary glomerular diseases. There was a significant negative correlation between proteinuria and capillary hemorrhage.