Different strategies have been proposed for the cardiovascular risk management of patients with rheumatoid arthritis (RA).
Objectives(1) To estimate the cardiovascular risk by different strategies in RA patients, analyzing which proportion of patients would be candidates to receive statin therapy; (2) to identify how many patients meet the recommended lipid goals.
MethodsA cross-sectional study was performed from a secondary database. The QRISK-3 score, the Framingham score (adjusted for a multiplying factor×1.5), the ASCVD calculator and the SCORE calculator were estimated. The indications for statin therapy according to NICE, Argentine Consensus, ACC/AHA, and new European guidelines were analyzed. The recommended LDL-C goals were analyzed.
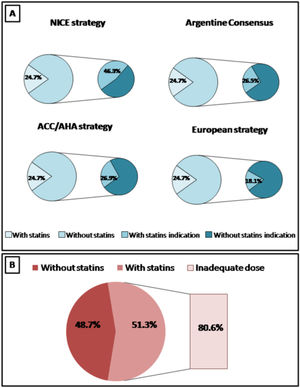
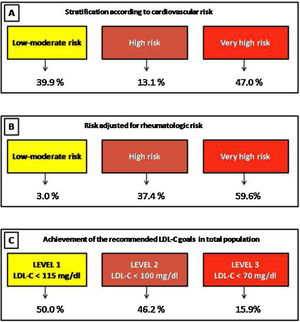
ResultsA total of 420 patients were included. In total, 24.7% and 48.7% of patients in primary and secondary prevention were receiving statins, respectively. Only 19.4% of patients with cardiovascular history received high intensity statins. Applying the ACC/AHA guidelines (based on ASCVD score), the Argentine Consensuses (based on adjusted Framingham score), the NICE guidelines (based on QRISK-3) and European recommendations (based on SCORE), 26.9%, 26.5%, 41.1% and 18.2% of the population were eligible for statin therapy, respectively. Following the new European recommendations, 50.0%, 46.2% and 15.9% of the patients with low-moderate, high or very high risk achieved the suggested lipid goals.
ConclusionApplying four strategies for lipid management in our population, the cardiovascular risk stratification and the indication for statins were different. A significant gap was observed when comparing the expected and observed statin indication, with few patients achieving the LDL-C goals.
Se han propuesto diferentes estrategias para el manejo del riesgo cardiovascular en pacientes con artritis reumatoide (AR).
Objetivos(1) estimar el riesgo cardiovascular mediante diferentes estrategias en pacientes con AR, analizando qué proporción de pacientes deberían recibir estatinas; (2) identificar cuántos pacientes alcanzaron los objetivos lipídicos recomendados.
MétodosEstudio de corte transversal. Se estimaron los puntajes QRISK-3, Framingham (ajustado por un factor multiplicador × 1,5), ASCVD y SCORE. Se analizaron las indicaciones de estatinas, según las guías NICE, el Consenso Argentino, las guías ACC/AHA 2018 y las nuevas directrices europeas. Se analizaron los objetivos de C-LDL.
ResultadosSe incluyeron 420 pacientes; 24,7 y 48,7% de los pacientes en prevención primaria y secundaria recibían estatinas, respectivamente. El 19,4% de los pacientes con antecedentes cardiovasculares recibían estatinas de alta intensidad. Aplicando las guías ACC/AHA (basadas en el puntaje ASCVD), el Consenso Argentino (basado en el puntaje ajustado de Framingham), las pautas NICE (basadas en el QRISK-3) y las recomendaciones europeas (basadas en el SCORE), 26,9, 26,5, 41,1 y el 18,2% de la población eran elegibles para el tratamiento con estatinas, respectivamente. Siguiendo las nuevas recomendaciones europeas, 50, 46,2 y 15,9% de los pacientes con riesgo bajo-moderado, alto o muy alto lograron los objetivos lipídicos recomendados.
ConclusiónAplicando varias estrategias para el manejo de los lípidos en nuestra población, la estratificación del riesgo cardiovascular y la indicación de estatinas fueron diferentes. Se observó una brecha significativa entre la indicación de estatinas esperada y observada, logrando los objetivos de C-LDL muy pocos pacientes.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation with a prevalence of 0.5–1% in the adult population.1 It is characterized by excess morbidity and mortality from cardiovascular disease.2–4
Multiple mechanisms have been proposed to explain the increased cardiovascular risk observed in the RA population.5,6 An increased prevalence of traditional cardiovascular risk factors is found in RA patients. In addition, the inflammatory process through mediators such as cytokines implicated in RA would play an important role in the development of atherosclerosis. Likewise, the action of autoantibodies [i.e. anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF)] on the arterial wall would also explain in part the increased cardiovascular risk.
However, the estimation of cardiovascular risk in subjects with RA has some peculiarities.7 The classical risk scores used to calculate cardiovascular risk have limitations, due to the fact that they were not developed specifically for RA. Most traditional cardiovascular risk prediction models developed for the general population do not include non-traditional cardiovascular risk factors such as autoantibodies or systemic inflammation. Then, when applied to patients with RA these classic scores have been found to significantly underestimate the true risk of cardiovascular disease. Inadequate screening for risk factors or cardiovascular disease would aggravate the situation. The low frequency of statins indication (or the administration of a lower dose) may be the result of this poor risk assessment.
Several strategies have been proposed to optimize cardiovascular risk stratification in RA subjects. One of them, recommended by the Consensus of Argentine Society of Cardiology and the European League Against Rheumatism (EULAR), is to adjust the risk calculated by a multiplying factor (1.5×) and follow the general recommendations for statin therapy.8,9 Following The National Institute for Health and Care Excellence (NICE) recommendations, another option is to use the QRISK-3 score, which includes in addition to traditional risk factors, the history of RA.10 Considering RA as a clinical situation that favors the indication of statins in intermediate risk subjects is proposed by a third strategy recommended by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for cholesterol management.11 Finally, a recent position paper of the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology (ESC) introduces for the first time a new algorithm for estimation of cardiovascular risk in RA patients and sets therapeutic goals for low-density lipoprotein cholesterol (LDL-C).12
Therefore, the objectives of the present study were: (1) to estimate the cardiovascular risk by different strategies in patients with RA; (2) to analyze which proportion of patients would be candidates to receive statin therapy and determine the reasons why they should receive them according to different strategies; (3) to identify how many patients meet the recommended lipid goals according to ESC algorithm.
Material and methodsA cross-sectional study from a secondary database was performed. All patients older than 18 years with a diagnosis of RA (fulfilling ACR/EULAR 2010 criteria)13 from January 1st, 2010 to December 30th, 2019 were included. Date of diagnosis was considered when first fulfilling ACR/EULAR 2010 criteria. For those patients diagnosed previously to this criteria publication date, we retrospectively reviewed their medical history identifying date of criteria fulfillment. The sample was obtained from two university hospitals and a network of 21 associated peripheral centers distributed in Buenos Aires City, Argentina. The medical histories of the patients included were revised, obtaining information about RA characteristics, cardiovascular risk factors and medication received at the time of the last registered rheumatologic visit.
Colorimetric and turbidimetric assays were used to measure non-fasting plasma levels of triglycerides, cholesterol bound to high-density lipoproteins (HDL-C) and total cholesterol. The Friedewald equation was used to calculate LDL-C. The blood levels of glucose, creatinine, glycated hemoglobin A1C and estimated glomerular filtration rate (eGFR) were measured according to standardized tests. All patients performed the tests in a single laboratory with the same methodology.
Familial hypercholesterolaemia was diagnosed using clinical characteristics (Dutch score ≥8 points). Moderate or severe chronic renal failure was defined when eGFR was between 30 and 59 or less than 30ml/min/1.73m2, respectively. Diabetes was diagnosed by one of the following criteria: fasting blood glucose level≥126mg/dl, oral glucose tolerance test (2h)>200mg/dl or glycated hemoglobin A1C test≥6.5% high blood pressure was diagnosed when systolic blood pressure was ≥140mmHg or diastolic blood pressure was ≥90mmHg. A history of cardiovascular disease was considered when the patient had coronary disease (acute myocardial infarction, unstable angina, stable chronic angina, myocardial revascularization surgery, coronary angioplasty), stroke, or peripheral arterial disease.
Four cardiovascular 10-years risk scores were calculated in subjects without cardiovascular disease:
- 1.
The QRISK-3 score estimates the risk of atherosclerotic cardiovascular events used by NICE guidelines, defining the population “at risk” when the calculated risk is ≥10%.10,14
- 2.
The Framingham score for atherosclerotic cardiovascular disease events, defining low, moderate, and high risk as values <10%, between 10% and 19%, and ≥20%, respectively.15 The risk values calculated by the Framingham score ware adjusted by a multiplication factor of 1.5, following the Argentine recommendations.9
- 3.
The atherosclerotic cardiovascular disease (ASCVD) calculator used by the ACC/AHA guidelines, defining low, borderline, moderate and high risk as values <5%, between 5% and 7.5%, between 7.5% and 19% and ≥20%, respectively.11
- 4.
The Systematic Coronary Risk Evaluation (SCORE) estimates the risk of cardiovascular fatal events used by the 2019 ESC guidelines. Risk <1%, between 1 and 4.9%, 5 and 9.9% or ≥10% was classified as low, moderate, high or very high, respectively.16
Applying recommendations of NICE guidelines, patients with a QRISK-3≥10% should receive statins.14 Also statins should be indicated in patients with chronic renal failure (eGFR less than 60ml/min/1.73m2), familial hypercholesterolemia or type I diabetes, despite their calculated risk score.
Considering the Argentine Consensuses, the following patient groups were considered candidates for statin therapy: (a) patients with diabetes; (b) patients with an LDL-C level >190mg/dl (with or without familial hypercholesterolemia); (c) subjects with a calculated Framingham score ≥20%; (d) patients with a calculated Framingham score ≥10% and <20% with one or more risk factors; (e) subjects with moderate to severe chronic renal insufficiency (eGFR between 30 and 59 or less than 30ml/min/1.73m2, respectively) without hemodialysis.9
Applying the 2018 ACC/AHA guidelines, the following patient groups were considered candidates for statin therapy: (a) patients 40–75 years of age with diabetes and LDL-C ≥70mg/dl; (b) patients 20–75 years of age with an LDL-C level >190mg/dl; (c) adults 40–75 years of age without diabetes and ASCVD risk ≥20%; (d) adults 40–75 years of age without diabetes and ASCVD risk of 7.5–19.9% (intermediate risk) with a risk-enhancing factors. Risk-enhancing factors include RA.11
Following the ESC position paper, the population was classified into 4 risk categories: low risk (SCORE <1%); moderate risk (SCORE ≥1% and <5%); high risk (SCORE ≥5% and <10%, diabetes without target organ damage or other major cardiovascular risk factor, moderate chronic kidney disease, markedly elevated single cardiovascular risk factor) and very high risk (SCORE ≥10, diabetes with target organ damage or other major cardiovascular risk factor, severe chronic kidney disease or history of cardiovascular disease).12 Additionally, the proposed algorithm by this Working Group stratifies RA according to RA-related characteristics influencing cardiovascular risk. “Low-risk RA” (LR-RA) is defined as seronegative, non-erosive RA in patients without extra-articular manifestations, in long-term (>1 year) remission (CDAI≤2.8 or SDAI≤3.3 or DAS28-ESR≤2.6), without active arthritis or persistently elevated C-reactive protein or erythrocyte sedimentation rate, with well-preserved physical function (HAQ-DI≤0.5), without high cumulative disease activity, not currently using glucocorticoids and without high cumulative glucocorticoid dose. All other patients are classified as “High-risk RA” (HR-RA). Patients with cardiovascular risk-enhancing RA-related factors (HR-RA) should be considered as having cardiovascular risk-category that is one level higher compared to the general population. For the low and moderate risk categories the recommended LDL-C goal is <115mg/dl. If the patient is classified as “HR-RA”, the proposed LDL-C goal is <100mg/dl. For the high-risk category, the recommended LDL-C goal is <100mg/dl. If the patient is classified as “HR-RA”, the proposed LDL-C goal is <70mg/dl. Finally, for the very high-risk category the recommended LDL-C goal is <70mg/dl.
Following the recommendations of all strategies, we consider for the present analysis that patients in secondary prevention should receive high intensity statins. It was defined as high intensity statins if they were able to reduce ≥50% the LDL-C level (atorvastatin 40–80mg or rosuvastatin 20–40mg per day).
Finally, those patients already receiving statin therapy were considered as subjects with appropriate prescription according to all guidelines.
Statistical analysisContinuous data were compared between groups using the t test for normal distribution or the Mann–Whitney–Wilcoxon test for non-normal distribution. The analysis of categorical data was performed using the chi-square test. Continuous variables are given as mean±standard deviation or median [25–75 interquartile range (IQR)] according to the distribution of the variables, while categorical variables are given as percentages. The agreement between different lipid management strategies in selecting patients with statin indication was analyzed, using the Cohen kappa index. Mild, discrete, moderate, significant, or almost perfect agreement was defined if the kappa value was <0.20, between 0.21 and 0.40, 0.41 and 0.60, 0.61 and 0.80, and 0.81 and 1, respectively. A value of p<0.05 was considered statistically significant. STATA 13.0 software packages were used for statistical analysis.
ResultsA total of 420 patients (mean age 69.7±13.8 years, 85.5% women) were included in the study. Globally, the prevalence of type 2 diabetes mellitus in the population was 7.6% and 51.4% of patients were hypertensive. The prevalence of current smoking was 6.4% and 18.1% of the population showed history of cardiovascular disease. The average time from RA diagnosis was 12.6±7.7 years. The baseline characteristics of the population are described in Table 1.
Characteristics of rheumatoid arthritis patients at the evaluation time.
| n=420 | |
|---|---|
| Continuous variables, mean (SD) | |
| Age, years | 69.7±13.8 |
| Systolic blood pressure, mmHg | 123.8±14.8 |
| Total cholesterol, mg/dl | 190.0±37.3 |
| LDL-C, mg/dl | 114.4±31.9 |
| HDL-C, mg/dl | 55.6±12.8 |
| Triglycerides, mg/dl | 116.7 (54.0) |
| Body mass index, kg/m2 | 26.5 (5.5) |
| Time from rheumatoid arthritis diagnosis, years | 12.6 (7.7) |
| CDAI | 7.4 (7.1) |
| SDAI | 7.9 (9.9) |
| DAS28 | 2.9 (1.2) |
| HAQ | 0.8 (0.6) |
| Categorical variables, % | |
| Female gender | 85.5 |
| Current smokers | 6.4 |
| Arterial hypertension | 51.4 |
| History of vascular disease | 18.1 |
| Type 2 diabetes mellitus | 7.6 |
| Chronic renal insufficiency | 10.0 |
| Atria fibrillation | 4.3 |
| Obesity | 22.3 |
| Therapy | |
| Corticosteroids | 46.9 |
| Conventional synthetic DMARDs | 72.6 |
| Biological DMARDs | 30.7 |
| Statins | 29.0 |
| Aspirin | 14.0 |
| Antihypertensive therapy | 47.9 |
| Antidiabetic therapy | 6.2 |
| Extra articular manifestations | 9.8 |
| Erosive disease | 25.2 |
| Positive cyclic citrullinated peptide | 81.9 |
| Positive rheumatoid factor | 66.6 |
The median adjusted Framingham score was 12.0% (IQR 8.0–21.9%). According to Argentinean Consensus, 22.1%, 23.7%, and 54.2% of the population was classified at low, moderate, or high risk. Likewise, the median ASCVD risk calculator was 8.2% (IQR 3.1–12.3%), and 20.6%, 6.9%, 27.0%, and 45.5% of the subjects were classified at low, borderline, moderate, or high risk according to AHA/ACC guidelines. The median QRISK-3 values were 17.6% (IQR 9.5–29.4%) and 79.2% of subjects were classified “at risk” following the recommendations of the NICE guidelines. The median SCORE risk was 1.0% (IQR 0.5–1.0%).
In total, 88.4% were considered HR-RA patients. Patients with high rheumatological risk were younger (68.9 vs. 75.4 years, p=0.002), although no significant differences were observed in the prevalence of high blood pressure (51.5% vs. 54.2%, p=0.72), current smoking (6.8% vs. 2.1%, p=0.38), diabetes (8.2% vs. 4.2%, p=0.33) or obesity (22.7% vs. 18.6%).
When considering cardiovascular and rheumatological risks according to the ESC guidelines, 3.0%, 37.4%, and 59.6% of patients were stratified at low-moderate, high, or very high risk, respectively.
Increased use of statins was observed in patients in secondary prevention, compared to subjects in primary prevention (48.7% vs. 24.7%, p<0.001). However, only 19.4% of patients with cardiovascular history received high intensity statins.
In those RA patients not taking statins, several strategies in cardiovascular prevention were analyzed. Applying the ACC/AHA guidelines (based on ASCVD score) and the Argentine Consensuses (based on adjusted Framingham score), 26.9% and 26.5% of the population were eligible for statin therapy, respectively.
According to the NICE guidelines and based on the QRISK-3 score, the use of statins was recommended in 41.1% of cases. When the ESC guidelines were applied (based on SCORE risk calculator and rheumatological risk), statins were recommended in 18.2% of patients.
The graphical representation of expected and observed statin use by four strategies is shown in Fig. 1.
The concordance between four strategies in selecting patients with statin indication was variable (Table 2).
Agreement in the indication of statins between the different strategies evaluated.
| NICE | ACC/AHA | ESC | Argentine consensus | |
|---|---|---|---|---|
| NICE | 0.58 | 0.13 | 0.51 | |
| ACC/AHA | 0.58 | 0.22 | 0.82 | |
| ESC | 0.13 | 0.22 | 0.30 | |
| Argentine consensus | 0.51 | 0.82 | 0.30 |
Concordance was evaluated using the kappa index.
ACC/AHA: American College of Cardiology/American Heart Association; ESC: European Society of Cardiology; NICE: The National Institute for Health and Care Excellence.
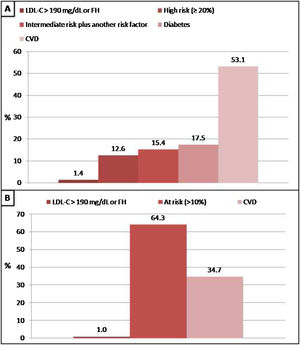
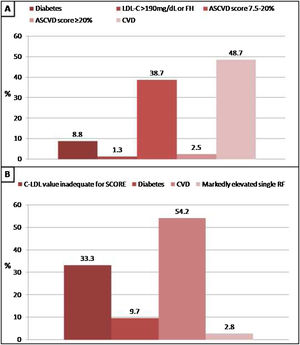
In patients without lipid-lowering treatment, the main reason for the indication of statin treatment was the history of cardiovascular disease when we applied Argentine Consensuses, ESC guidelines or ACC/AHA recommendations. However, the main reason for the indication of statin treatment was the “at risk” categorization of the population when we applied NICE guidelines. The reasons why statin therapy should be indicated using different strategies are shown in Figs. 2 and 3.
Reasons why patients without lipid-lowering treatment would have an indication of statins according to the Consensus of the Argentine Society of Cardiology (SAC) and The National Institute for Health and Care Excellence (NICE) guidelines. A: SAC, B: NICE. CVD: cardiovascular disease; FH: familial hypercholesterolaemia.
Reasons why patients without lipid-lowering treatment would have an indication of statins according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines and recommendations of European Society of Cardiology (ESC). A: ACC/AHA, B: ESC. CVD: cardiovascular disease; FH: familial hypercholesterolaemia; RF: risk factor.
Finally, following the recommendations of the Working Group of the ESC, and considering the cardiovascular and rheumatological risks estimated, we analyzed the achievement of the recommended LDL-C goals in the total population (treated or not with statins). Then, 50.0%, 46.2% and 15.9% of the patients with low-moderate, high or very high risk achieved the suggested lipid goals (Fig. 4).
DiscussionThis work evaluated four different strategies for cardiovascular stratification and statin indication in a population with RA. Also, we analyzed the application of a new algorithm that established LDL-C goals for the first time in this population.
All current guidelines on the prevention of cardiovascular disease in clinical practice recommend the assessment of cardiovascular risk. Patients with a higher cardiovascular risk should receive more intense interventions. However, cardiovascular risk algorithms developed for the general population are not accurate in RA patients. These predictive tools were not specifically developed in patients with RA and the performance is suboptimal because traditional cardiovascular risk factors do not fully explain the increased cardiovascular risk. Inflammation contributes to atherosclerosis plaque vulnerability and clinical atherothrombotic events, but current risk functions do not represent this contributing factor. Consequently, cardiovascular risk is often underestimated.17
Various strategies have been proposed to deal with this problem. One of them uses a correction factor to adjust the estimated risk. Another of them includes in addition to the variables commonly used in most risk scores, the history of RA. Considering RA as a potentiating factor in intermediate risk subjects is proposed by a third strategy. Finally, establishing cardiovascular and rheumatological risks jointly to determine lipid goals is a novel recommendation. In this study, we evaluate all the different strategies proposed.
Our data shows that cardiovascular stratification varies according to the strategies used. However, the proportion of subjects classified as “at risk”, high risk or very high risk was considerable, regardless of the strategy used. Previous reports have shown that the concordance between the different scores was variable in this population.18 Similarly, Jafri et al. showed that approximately 10% of RA subjects had discordant 10-year cardiovascular risk scores when comparing the Framingham score with ACC/AHA score.19 These findings did not significantly change when a 1.5 multiplier was applied to the Framingham score.
Strong evidence shows that the reduction of LDL-C using statin treatment leads to a significant decrease in the cardiovascular risk. However, the evidence in RA patients is limited. A post hoc analysis of two prospective trials of statins showed that those subjects with and without inflammatory joint disease experience comparable lipid-lowering effects and cardiovascular risk reduction after intensive treatment with statins.20 Likewise, Schoenfeld et al. conducted an incident user cohort study with time-stratified propensity score matching using a general population database.21 In this study, statin initiation was associated with a 21% lower risk of all-cause mortality. In the same way, Rollefstad et al. showed that intensive lipid-lowering treatment with rosuvastatin induced atherosclerotic regression in patients with inflammatory joint disease.22
A single randomized clinical trial developed to assess the impact of statins on cardiovascular risk in RA patients has been published to date.23 Atorvastatin 40mg daily was safe and results in a significantly greater reduction of LDL-C level than placebo. The unexpectedly low event rate and resulting limited statistical power to detect cardiovascular end-points effect during the planned 5 years of follow up led to premature termination of the trial.
The statin indication also varied according to the analyzed strategy. The greatest indication was observed when applying the NICE guidelines (about half), followed by the Argentine and North American recommendations (about a quarter) and the European guidelines (about a fifth). The agreement between the different strategies in selecting subjects with an indication of statins was highly variable. The best concordance was observed between the Argentine Consensus and the ACC/AHA guidelines and the worst agreement was demonstrated between the European recommendations and the NICE guidelines.
Tournadre et al. calculated the proportion of patients eligible for statins according to ESC guidelines, the Adult Treatment Panel III (ATP-III), and the ACC/AHA in a French cohort of statin-naïve RA patients.24 A marked discordance in risk assessment and cholesterol treatment was observed between the three sets of guidelines. Similarly, another small Mexican study showed fair statin eligibility agreement (k=0.242) between ACC/AHA 2013 and ATP-III guidelines.25 Another study showed that in a group of 335 patients with inflammatory joint diseases (including 201 subjects with RA), 183 and 159 patients had a calculated cardiovascular risk by SCORE and ACC/AHA <5%, indicating no need of lipid lowering treatment.26
These findings were reported in other chronic inflammatory diseases. Masson et al. showed that not all patients with psoriasis should receive statins.27 The lipid-lowering therapy indication was similar when applying the ACC/AHA and ESC guidelines but the concordance was moderate. On the other hand, another study reported that a large proportion of patients with systemic lupus erythematosus should receive statins after applying the strategies based on the Framingham score and the QRISK-3.28
In the present study, when analyzing patients who were not receiving lipid-lowering therapy, the main reason for the indication of statin treatment was the history of cardiovascular disease. However, in subjects in primary prevention, the presence of diabetes or moderate-high cardiovascular risk (calculated with the scoring method) were also important reasons for its recommendations.
The position paper of the Working Group of the ESC introduces for the first time a new algorithm for estimation of cardiovascular risk (considering cardiovascular and rheumatological risk) in RA patients and sets therapeutic goals for LDL-C.12 Our results showed that the majority of RA patients were classified as “high-risk rheumatology”. Consequently, a large number of patients were reclassified, establishing lower LDL-C goals. Therefore, a great proportion of patients did not achieve the recommended lipid goals.
Age is a determining factor when estimating cardiovascular risk. Consequently, cardiovascular risk is frequently underestimated in younger patients. Our study showed that the age was lower in HR-RA patients compared to LR-RA patients. Therefore, many young patients with RA, despite showing characteristics of the disease that place them in a category of higher cardiovascular risk, would be classified as “low risk” if we only applied tools based on traditional risk factors. The low frequency of statins indication may be the result of this deficient risk assessment. This fact constitutes a major point of concern in RA, a condition associated with early atherosclerotic disease. In this sense, one study found that RA significantly elevated the risk of cardiovascular disease in the young population, relative to non-RA controls.29 Even without comorbidity at baseline, patients with RA still had a greater risk. Similarly, a meta-analysis showed that the rate of cardiovascular events was significantly higher in young patients with RA compared with controls.30 We consider that a comprehensive evaluation of young patients, considering the classical risk factors and the “rheumatological risk” constitutes the best option.
In everyday practice a number of patients with RA do not receive adequate treatment regardless of developed atherosclerosis. Unfortunately, despite high risk for cardiovascular disease mortality, screening and treatment of hyperlipidemia in patients with RA is suboptimal.31
Guideline recommended treatment to targets of cardiovascular risk is inadequate in patients with inflammatory joint diseases. A study that included 2277 patients with chronic inflammatory diseases (1376 with RA) showed that the lipid-lowering treatment and antihypertensive medication were only indicated in 36.1% and 52.6% of cases, respectively.32 The LDL-C and blood pressure targets were obtained in 26.2% and 26.3%, respectively. Guideline recommended treatment and/or corresponding treatment targets were not initiated or obtained in approximately 50%. Another study showed that one of the main barriers for rheumatologists to manage cardiovascular risk in RA subjects is lack of time.33 Rheumatologists also perceived conflict regarding of hyperlipidemia management and that they lack training and knowledge of hyperlipidemia guidelines.34 Similarly, some studies demonstrated low patient awareness of cardiovascular risk with RA and low rates of patient-reported counseling by physicians.35
In addition to optimal management of the underlying inflammatory condition according to current guidelines, individual cardiovascular risk factors, particularly dyslipidaemia, should be assessed regularly and guide risk stratification and requirement for treatment. In our opinion it is necessary to reach an agreement between rheumatologists, cardiologists and general practitioners for screening of cardiovascular diseases in patients with RA.
ConclusionStratification of cardiovascular risk in RA patients can be a real challenge. Applying four strategies for lipid management in our population, the indication for statins was considerably different. However, a significant gap was observed when comparing the expected and observed statin indication, with very few patients achieving the LDL-C goals. Interdisciplinary work between rheumatologists, cardiologists and clinicians could improve these results.
Authors’ contributionsMasson Walter was the main coordinator of the project and was responsible for the study design. Masson Walter, Rossi Emiliano and Scolnik Marina drafted the manuscript of the present paper. Cornejo-Peña Guillermo, Mora-Crespo Lorena, Fiorini Norberto, Alvarado Rodolfo, Damonte Juan and Tobar-Jaramillo Mayra were involved in the supervising of data collection and stratification. Masson Walter and Rossi Emiliano contributed to data assembly and analysis. Scolnik Marina and Masson Walter contributed with manuscript revision. All authors contributed intellectually to this manuscript and have approved this final version.
Ethics approvalThe study was conducted in compliance with the recommendations for medical research. The protocol was reviewed and approved by the Ethical Board of the Institution.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestNone.