Systemic lupus erythematosus (SLE) is an inflammatory autoimmune systemic and chronic disease. Fertility in SLE patients is considered normal; factors that have been associated in these patients with ovarian failure are: disease activity, autoantibodies, and the use of cytotoxic agents. The anti-Müllerian hormone (AMH) is a marker that helps to determine the follicular reserve.
ObjectiveThe objective was to determine AMH levels in women of reproductive age with SLE.
Material and methodsWe included 65 women with SLE classified according to the 1997 ACR criteria, 18–40-years old. We obtained demographic, clinical, obstetric, and gynecological characteristics as well as serum levels of AMH. We performed a bivariate analysis among patients with low ovarian reserve and those with normal ovarian reserve. We also performed a correlation analysis between activity and damage index and between the cumulative cyclophosphamide dose and AMH levels.
ResultsWe found a median of serum AMH in SLE patients of .61ng/ml. The prevalence of low ovarian reserve in our study was 3.07%. We found a median MEX-SLEDAI score of 1 point and the median SLICC score was 2 points. Twenty-five patients (38.4%) had used cyclophosphamide and their cumulative average dose was 7.5g.
ConclusionsWe found a median of AMH of .61ng/ml in our population. The prevalence of low ovarian reserve in SLE patients was 3.07%. We did not find a correlation between AMH levels, the use of cyclophosphamide, and disease activity.
El lupus eritematoso sistémico (LES) es una enfermedad autoinmune inflamatoria sistémica crónica; se considera que la fertilidad es normal en pacientes con LES, los factores asociados con una baja reserva folicular que condicionan falla ovárica son: actividad de la enfermedad, anticuerpos antiovario y el uso de agentes citotóxicos. La hormona anti-mülleriana (HAM) es un marcador para determinar la reserva folicular.
ObjetivoDeterminar los niveles de HAM en mujeres con LES en edad reproductiva.
Material y métodosIncluimos a 65 mujeres, de 18 a 40 años, clasificadas como LES según los criterios ACR 1997. Se obtuvieron las características demográficas, clínicas, ginecoobstétricas y niveles séricos de HAM. Se realizó un análisis bivariado entre las pacientes con baja reserva ovárica y aquellas con reserva ovárica normal. Se realizó un análisis de correlación entre los índices de actividad y daño, así como la dosis acumulada de ciclofosfamida y los niveles de HAM.
ResultadosLa mediana del título de HAM fue de 0,61ng/ml. La prevalencia de baja reserva ovárica en nuestro estudio fue del 3,07%. La mediana del MEX-SLEDAI fue de 1 punto y la de SLICC 2 puntos. Veinticinco pacientes (38,4%) habían utilizado ciclofosfamida y la dosis promedio acumulada fue de 7,5g.
ConclusionesEn nuestra población, se encontró una mediana del título de HAM de 0,61ng/ml, similar a lo reportado anteriormente. La prevalencia de baja reserva ovárica fue del 3,07%. No se encontró correlación entre el uso de ciclofosfamida, la actividad de la enfermedad o los niveles de HAM.
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune inflammatory disease caused by autoantibodies, characterized by remissions and exacerbations. SLE can occur at any age, although its peak incidence is between 15 and 44 years of age, within the reproductive period of women. Therefore, obstetric disease and altered fertility are important.1,2 Although it has been described that fertility is normal in most patients with SLE,3 this can be affected by each patient's ovarian reserve. Ovarian failure by low hormone ovarian reserve has, as aggravating factors, the disease activity measured by SLEDAI, the presence of antiovarian antibodies4 and the use of cytotoxic agents.5 Of the latter group, cyclophosphamide is the most studied prodrug due to its gonadal toxicity, damage to the ovarian follicles, follicular maturation and depletion of primordial follicles.5–9
In 2002, studies were initiated on the clinical application of determination of serum anti-Mullerian hormone (AMH), making it a marker with high sensitivity and specificity to determine the follicular reserve.10,11 This hormone, also called Mullerian inhibitory substance, belongs to the superfamily of transforming growth factor β, is a dimeric glycoprotein with two disulfide bonds, with a molecular weight of 140kDa and is mainly expressed in the granulosa cells.12–18 Serum AMH levels correlate with the development of preantral and small antral follicles during reproductive life.19
The AMH has been studied in different populations.13,20–23 It is known that AMH levels are not altered in women using hormonal contraceptives.24 Some studies have evaluated AMH levels in patients with cancer and chemotherapy.17,25,26 Retrospective studies have evaluated the prevalence of ovarian failure in SLE patients treated with cyclophosphamide, determining ovarian failure based on the presence or absence of amenorrhea, which is very sensitive and specific. More recently AMH has been used for measurement in SLE patients with and without immunosuppressants and a relationship has been found with the use of cyclophosphamide, but not with the use of other immunosuppressive agents.27,28 It has also been studied in Juvenile lupus29 and a downward trend in the parameters of ovarian reserve was seen, though not statistically significant, related to the use of cyclophosphamide, even when29 patients were still menstruating. AMH measurements were also used to assess the role of gonadotropins’ agonists in preventing ovarian failure associated with cyclophosphamide.30 It has been reported that AMH may be decreased in SLE patients who never received cyclophosphamide compared with controls, indicating that fertility is itself affected by SLE per se.31
There may be clinical differences in SLE patients of different ethnic backgrounds.32 In the multicenter LUMINA LVIII study, predictors of premature gonadal failure were evaluated in 316 patients with SLE, which was defined by amenorrhea for >6 months and/or menopause before age 40; the study enrolled Hispanic patients, and found a prevalence of 11.7% of developing premature gonadal failure and it was established that the presence of high disease activity, Texas Hispanic ethnicity, and previous use of cyclophosphamide and older age were predictors of premature gonadal failure.33
There is the possibility that differences are not only due to social factors, but molecular and genetic factors only may modify the usefulness of this measurement in our population. For this reason, we studied AMH levels among Mexican women of reproductive age with SLE to assess factors associated with low ovarian reserve.
Material and MethodsAn analytical cross-sectional study from March to August 2011 was performed. We consecutively included 65 women aged 18–40 years, diagnosed with SLE, who fulfilled at least 4 classification criteria of the American College of Rheumatology (ACR), revised in 1997 for SLE,34 from the Rheumatology Department, Hospital Universitario “Dr. José Eleuterio González” in Monterrey, Nuevo Leon, Mexico. We excluded patients with overlap syndromes, and established diagnosis of ovarian failure and those with a history of ovarian surgery.
After signing informed consent, demographic (age, education level), clinical (disease duration, weight, height, body mass index [BMI], smoking, drug use) and obstetric characteristics (pregnancies, births were obtained abortions, cesarean sections and age at menarche) were collected.
They were given a questionnaire to assess the damage index due to lupus by the Systemic Lupus International Collaborating Clinic (SLICC) validated in Spanish, which was determined in ranges from 0 to 46 points, with activity defined as >4 points.35,36 The MEX-SLEDAI index was used to evaluate the activity of the disease, which contains 24 specific variables grouped by systems with a maximum of 32 points and which are divided, according to the score, in the inactive condition, mild, moderate or severe activity.37
We performed measurements of serological variables included hemoglobin (normal range 12–14g/dl) and erythrocyte sedimentation rate (ESR) as well as immunological variables: antinuclear antibodies (ANA) by indirect immunofluorescence performed with Hep2 cells (negative<1:40), anti-dsDNA antibodies determined by indirect immunofluorescence with Crithidia luciliae (negative<1:20), anti-Sm antibodies determined by ELISA (negative<15U/ml), anticardiolipin IgM and IgG determined by ELISA (negative<11 UMPL and <23 UGPL, respectively), complement levels by nephelometry (C3: 50–120mg/dl, C4: 20–50mg/dl) and lupus anticoagulant by coagulometric method with citrated plasma (Weak: 1.2–1.5/s, moderate: 1.5–2.5/s, strong>2/s).
Serum AMH levels were measured by duplicate in venous blood using ELISA (human anti-müllerian hormone, ELISA Kit TSZ Scientific LLC-Biotang Inc.) using reagents and calibrators provided by the manufacturer. A value <0.35ng/ml was used to define low ovarian reserve, according to Almog et al.13
A database was created in Excel and statistical analysis was done using the SPSS® version 19 statistical program. Quantitative variables were expressed as mean±SD or median and interquartile range, depending on the Gaussian distribution with the Kolmogorov–Smirnov test. The comparative analysis was done for normal quantitative variables with Student's t or Mann–Whitney's test otherwise.
Categorical variables were compared using chi-square or Fisher's exact test, according to the observations distributed in a cuadricelular table. The protocol was registered by the Ethics Committee of the Division of Research of the Faculty of Medicine and the University Hospital “Dr. José Eleuterio González” of the Autonomous University of Nuevo León.
ResultsSixty-five women were included in the study, with a mean age±SD of 28.6±6.4 years and a mean disease duration of 5.6±4.5 years. The average BMI was 25±5.9kg/m2. Regarding the patient's profession, 43 (66.2%) were unskilled workers, 12 (18.5%) university trained professionals, 9 (9.2%) had technical studies, and 3 (4.6%) (1.5%) were skilled workers. Regarding his educational level, 33 (50.8%) studied secondary school completely, 22 (33.8%) a college career, 8 (12.3%) were illiterate and 2 (3.1%) had incomplete secondary studies. Eight (12.3%) were actively smoking. The drugs used at the time of the study were: prednisone 69.2%, hydroxychloroquine 52.3%, chloroquine 24.6%, methotrexate 20.0%, leflunomide 1.5%, sulfasalazine 1.5%, azathioprine 24.6%, mycophenolate mofetil 6.2%, rituximab 7.7% and cyclophosphamide 25 (38.4%) women, with a mean cumulative dose of 7.5±6g. The median activity index and MEX-SLEDAI fu 1 (IQR 0–3.5) and median SLICC damage index was 2 (IQR 1–3.5).
As for their reproductive background, median menarche was 12 years (IQR 11.5–13.5). Eleven patients (16.9%) had menstrual abnormalities, the median number of pregnancies was 1 (IQR 0–2) and only 4 patients (6.2%) had a history of preeclampsia. Thirty-one patients (47.6%) had no pregnancies.
As for paraclinical variables, mean hemoglobin was 12.1±1.5g, mean ESR was 24±15mm/H; ANA titers showed the following prevalences: 1:80 (38.5%); 1:160 (23.1%); 1:320 or more (7.7%) and were negative (16.9%). The predominant pattern was homogeneous in 47.7% of the titers, followed by speckled pattern in 15.4% and nucleolar pattern in 6.2%.Titers of anti-dsDNA antibodies were positive in 26.2%. Anti-Sm antibodies had a median of 6.1U/ml (IQR 4.4–9.6). The mean complement C3 fraction was 78±33mg% and C4 complement fraction of 22±11mg%. The mean level of IgM anticardiolipin antibodies was 4.3±4.0UMPL and for IgG was 8.5±7.5UGPL.
In our population, the median AMH level was 0.61ng/ml (IQR 0.49–0.74). The prevalence of low ovarian reserve in our study was 3.07% (2 patients). Fifteen patients were between 3 and 25 percentile (0.3879–0.4955ng/ml). There was no correlation between prior use of cyclophosphamide and AMH level (p=.88); in turn, the median gestations of patient exposed and not exposed to cyclophosphamide; when compared found 0 (IQR 1) and 1 (IQR 3), respectively, with no significant differences among the patients (p=.042).
No correlation between the SLE activity measured by MEX-SLEDAI and AMH levels was found; however, when the ratio of AMH levels and target organ involvement was assessed by SLICC, it was found that in the group with damage measured by SLICC (n=50) it was 0.6425 (IQR 0.28) vs the undamaged group (n=15), which was 0.5260 (IQR 0.16), with a statistically significant difference, with p=.03, using the U test of Mann–Whitney.
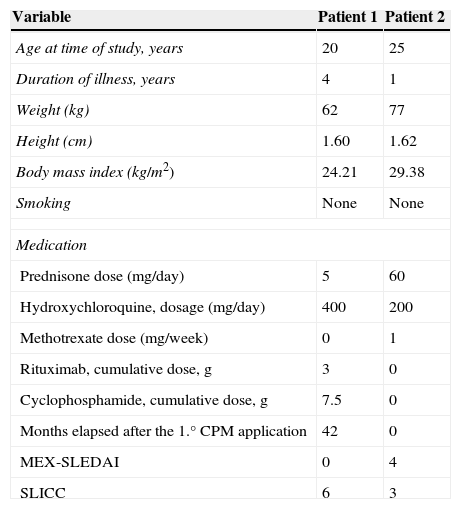
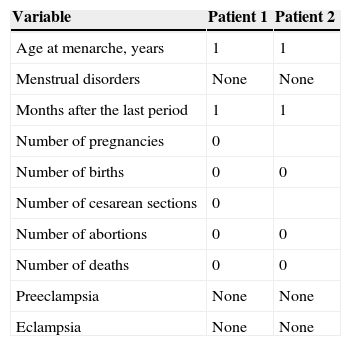
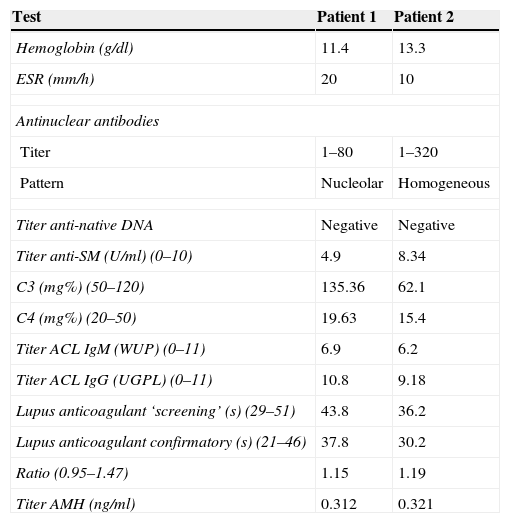
Clinical and demographic characteristics of patients with low ovarian reserve are described in Table 1, where we can see that the first patient with low reserve is 20 years of age, disease duration of 4 years, treated with a dose of prednisone 5mg/day, hydroxychloroquine 400mg/day, a cumulative dose of 3g rituximab, cyclophosphamide cumulative dose of 7.5g, with MEX-SLEDAI 0 and SLICC 6. The second patient with low ovarian reserve is 25 years old, one year since the diagnosis of the disease, treated with prednisone 60mg/day, hydroxychloroquine 200mg/day and methotrexate 15mg/week, received no biological or cyclophosphamide, MEX-SLEDAI was 4 and 3 SLICC. Regarding the obstetric data from both patients, none had menstrual disturbances, only the second patient had two pregnancies, the last one 5 years ago (Table 2). Regarding both patients’ paraclinical variables, no significant data was seen, all being negative, including anti-dsDNA, anti-Sm and anticardiolipin antibody; complement and lupus anticoagulant were normal. The title of the first patients’ AMH was 0.312ng/ml and in the second it was 0.321ng/ml (Table 3).
Demographic and Clinical Characteristics of Patients With Low Ovarian Reserve.
| Variable | Patient 1 | Patient 2 |
|---|---|---|
| Age at time of study, years | 20 | 25 |
| Duration of illness, years | 4 | 1 |
| Weight (kg) | 62 | 77 |
| Height (cm) | 1.60 | 1.62 |
| Body mass index (kg/m2) | 24.21 | 29.38 |
| Smoking | None | None |
| Medication | ||
| Prednisone dose (mg/day) | 5 | 60 |
| Hydroxychloroquine, dosage (mg/day) | 400 | 200 |
| Methotrexate dose (mg/week) | 0 | 1 |
| Rituximab, cumulative dose, g | 3 | 0 |
| Cyclophosphamide, cumulative dose, g | 7.5 | 0 |
| Months elapsed after the 1.° CPM application | 42 | 0 |
| MEX-SLEDAI | 0 | 4 |
| SLICC | 6 | 3 |
CPM: cyclophosphamide; MEX-SLEDAI: Mexican Systemic Erythematosus Disease Activity Index; SLICC: Systemic Lupus International Collaborating Clinics damage index.
Gynecological and Obstetric Data of Patients With Low Ovarian Reserve.
| Variable | Patient 1 | Patient 2 |
|---|---|---|
| Age at menarche, years | 1 | 1 |
| Menstrual disorders | None | None |
| Months after the last period | 1 | 1 |
| Number of pregnancies | 0 | |
| Number of births | 0 | 0 |
| Number of cesarean sections | 0 | |
| Number of abortions | 0 | 0 |
| Number of deaths | 0 | 0 |
| Preeclampsia | None | None |
| Eclampsia | None | None |
Paraclinical Variables of Patients With Low Ovarian Reserve.
| Test | Patient 1 | Patient 2 |
|---|---|---|
| Hemoglobin (g/dl) | 11.4 | 13.3 |
| ESR (mm/h) | 20 | 10 |
| Antinuclear antibodies | ||
| Titer | 1–80 | 1–320 |
| Pattern | Nucleolar | Homogeneous |
| Titer anti-native DNA | Negative | Negative |
| Titer anti-SM (U/ml) (0–10) | 4.9 | 8.34 |
| C3 (mg%) (50–120) | 135.36 | 62.1 |
| C4 (mg%) (20–50) | 19.63 | 15.4 |
| Titer ACL IgM (WUP) (0–11) | 6.9 | 6.2 |
| Titer ACL IgG (UGPL) (0–11) | 10.8 | 9.18 |
| Lupus anticoagulant ‘screening’ (s) (29–51) | 43.8 | 36.2 |
| Lupus anticoagulant confirmatory (s) (21–46) | 37.8 | 30.2 |
| Ratio (0.95–1.47) | 1.15 | 1.19 |
| Titer AMH (ng/ml) | 0.312 | 0.321 |
ACL: anticardiolipin; AMH: anti-Mullerian hormone.
This is the first study in our country that evaluates AMH levels in a group of Mexican patients with SLE, with and without cyclophosphamide, which gives importance to work, as this population has negative effects on ovarian reserve, which the can lead to fertility problems.
The behavior of AMH in patients without cyclophosphamide in our population is similar to that described in the nomogram of infertile women published by Almog et al.13 However, they are lower than those reported in the study by Lawrenz et al., which evaluated AMH levels in 33 women with SLE without prior treatment with cyclophosphamide, which may be explained by ethnic and age-based differences, and exclusion of patients with cyclophosphamide; plus the fact that the kit used to measure AMH is different from that used in our study.31 Recently, Morel et al. assessed the probability of pregnancy in 46 SLE patients under 40 years of age with and without exposure to cyclophosphamide compared with control subjects, which found that AMH levels were lower in patients exposed to the drug and in patients older than 30 years. In patients with SLE, 32 of the 38 pregnancies were successful and it was finally settled that the decrease in pregnancy rates is negatively influenced by exposure to cyclophosphamide and age, not AMH38 levels. We believe that the decline in fertility in our study (which was determined by the number of pregnancies among those exposed to cyclophosphamide or not) was influenced by medical advice to patients not to get pregnant and not secondary to the use of cyclophosphamide.
The utility of AMH measurement to determine ovarian reserves has been studied in control populations, in the study by Tremellen et al., which assessed 238 women. 16 patients had a low ovarian reserve (measured by AMH), with a sensitivity of 80% and a specificity of 85%, positive predictive value 67% and negative predictive value of 97%.39 In this study of patients with SLE, the prevalence of low ovarian reserve of 3.07% (2 patients) according to the cutoff value described by Almog et al.
In accordance with data described by Boumpas et al.,5 both the patient's age and the dose of cyclophosphamide can affect ovarian reserve, which is reflected in the level of AMH of the first patient, with diminished ovarian reserve, who had a 7.5gcumulative dose of cyclophosphamide.
However, in the total study population, we found no correlation between the cumulative dose of cyclophosphamide and level of AMH. The discrepancy with previous studies evaluating ovarian failure in SLE patients treated with cyclophosphamide intravenously5 is because they define ovarian failure based on more than 12 or 20 months of amenorrhea. Additional factors that can explain are the cumulative dose of cyclophosphamide and patient age, as it is described that the risk of ovarian involvement is dependent on dose and age.
The second patient with low ovarian reserve had not received cyclophosphamide, so low AMH levels could be explained by the presence of other associated factors, including the possible presence of genetic factors, or ovarian antibodies against AMH that could even interfere with the laboratory assay, which is to be determined in future studies. This is in line with that reported by Lawrenz et al., who mentioned that patients with SLE without cyclophosphamide have AMH at lower levels than controls.31
In previous years, regular menstrual periods were a readily ascertainable external parameter of ovarian function. Today, we know that the presence of regular periods is not synonymous with fertility, which can be reflected in the 2 patients with low ovarian reserve in our study who had regular menstrual cycles, something that is consistent with that reported.29
Recently, we have studied a subclinical worsening of ovarian reserve in patients with SLE. Ma et al. evaluated 3 groups of patients, all with regular menstrual cycles: SLE without use of cytotoxic drugs, SLE and use of cytotoxic drugs, and a control group, where it was found that AMH levels and antral follicle counts were lower in the 2 groups of SLE, compared with controls, finding no significant difference between them.40 In our population, we found no correlation between the index of SLE activity measured by MEX-SLEDAI and AMH levels, which can be explained because the SLE patients in our study had low disease activity measured by the MEX-SLEDAI; however, due to the characteristics of the sample size, we could not perform a statistical analysis by levels of disease activity.
Our study has several limitations. The first is that you cannot make direct comparisons with other populations, because the kit used was different than that from previous studies. However, the cutoff values in the 3rd percentile (<0.3287ng/ml) correlated with the values described. The second is that the number of subjects with cyclophosphamide was not paired with those who did not receive it, because this was not a case–control study. Finally, a control group was not used.
In conclusion, in our population the median AMH titer was 0.61ng/ml, IQR of 0.49–0.4a. The prevalence of low ovarian reserve in our study is 3.07% (2 patients). There was no correlation between the use of cyclophosphamide, SLE activity and the level of AMH.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this research did not perform experiments on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace on the publication of data from patients, and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FundingThis study used self-resources from the Rheumatology and an unconditional support grant of $60,000 Mexican pesos from the Mexican College of Rheumatology, AC. The author reports that he does not have any funding to declare.
Conflict of InterestThe authors have no disclosures to make.
To the Department of Gynecology and Obstetrics, University Hospital for their cooperation.
Please cite this article as: Velarde-Ochoa MdC, Esquivel-Valerio JA, Vega-Morales D, Skinner-Taylor CM, Galarza-Delgado DÁ, Garza-Elizondo MA. Hormona anti-mülleriana en mujeres en edad reproductiva con lupus eritematoso sistémico. 2015;11:78–82.