To analyse and compare changes in the collection of clinical variables after the implementation in daily practice of an evaluation checklist for patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA).
MethodsAn observational study was performed based on medical records review. The number and type of variables of the evaluation checklist in the medical records were collected. The first review was made before the implementation of the checklist, and the second one 6 months after the implementation (in different patients). A descriptive and bivariate analysis was carried out.
ResultsSix hospitals and 11 rheumatologists participated. A total of 83 and 68 medical records were reviewed before and after the implementation of the checklist. After the implementation, in the axSpA patients, a significant increase was recorded in alcohol consumption, diarrhoea or IBD and urethritis, diabetes mellitus, hyperlipidaemia, depression, obesity or gout/hyperuricaemia, weight, height, blood pressure, patient and physician global assessments of disease activity, BASDAI and DAS28. And, in the PsA patients, alcohol consumption, hypertension, diabetes mellitus, hyperlipidaemia, disease, gout/hyperuricaemia, thoracic expansion, cervical rotation, weight, height, blood pressure, patient and physician global assessments of disease, ASDAS, BASDAI, and BASFI were recorded. In general, there was a trend towards greater recording in axSpA compared with PsA.
ConclusionsThe implementation of a specific checklist in daily practice improves the evaluation of patients with axSpA and PsA. More efforts are necessary to continue improving the evaluation of patients with axSpA, but especially of those with PsA.
Analizar y comparar los cambios en la recogida de variables clínicas tras la implementación en la práctica diaria de un checklist de evaluación para pacientes con espondiloartritis axial (EspAax) y artritis psoriásica (APs).
MétodosSe realizó un estudio observacional. Mediante revisión de historias médicas, se recogieron el número y el tipo de variables del checklist de evaluación que figuraban en las mismas. La primera revisión se realizó antes de la implementación del checklist, y la segunda, 6 meses después de la implementación (pacientes diferentes) para poder comparar los cambios producidos con la misma. Se realizó un análisis descriptivo y bivariado.
ResultadosParticiparon 6 hospitales y 11 reumatólogos. Se revisaron un total de 83 y 68 historias médicas pre- y post-implementación del checklist. Tras la implementación, en la EspAax aumentó significativamente el registro en la historia clínica del consumo de alcohol, diarrea o enfermedad inflamatoria intestinal (EII) y la uretritis, diabetes mellitus, hiperlipidemia, depresión, obesidad o la gota/hiperuricemia, peso, talla, presión arterial, VGM, VGP, BASDAI y DAS28. Y en la APs el consumo de alcohol, HTA, diabetes mellitus, hiperlipidemia, enfermedad, gota/hiperuricemia, expansión torácica, rotación cervical, peso, talla, presión arterial, VGM, VGP, ASDAS, BASDAI y BASFI. Tanto pre- como post-implantación en general existe una tendencia a un mayor porcentaje de recogida de variables en pacientes con EspAax que en pacientes con APs.
ConclusionesLa implementación de un checklist específico en la práctica diaria mejora la evaluación de los pacientes con EspAax y APs. Se debe seguir trabajando en la mejoría de la evaluación de los pacientes con EspA, pero especialmente en la APs.
The spondyloarthritides (SpA) are a very heterogeneous group of diseases where suboptimal patient assessment has been observed in routine clinical practice.1–6 Thus, the EmAR II study, for example, showed that in the assessment of patients with SpA, approximately 60% of medical records did not include an evaluation of possible joint involvement.7 Similarly, 87% of the records also showed no joint index, and 84% did not show a functional index. Studies in other countries have described a similar scenario.3
The ONLY TOOLS study was designed with these data in mind, and created an assessment checklist for patients with SpA in order to standardise and more strictly monitor the disease and comorbidities, and to help identify high risk and response factors.8 Subsequently, the Práctica Madrid project was launched, in which the aforementioned checklist was implemented in routine clinical practice, and which demonstrated on its first analysis that it is feasible and that it significantly improves the assessment of patients with SpA.
On the other hand, although axial SpA (axSpA) and psoriatic arthritis (PsA) are included in the SpA group, they have different characteristics, even beyond joint involvement. Patients with PsA show a higher percentage of cardiovascular involvement, metabolic syndrome or cancer compared to other forms of SpA.9 However, these findings should not influence patient assessment, but should serve to reinforce their collection. Therefore, the aim of this sub-study was to analyse the baseline data regarding patient assessment and changes in data collection comparatively between patients with axSpA and PsA within the Práctica Madrid project.
MethodsDesignA sub-analysis of the Práctica Madrid study, endorsed by the Rheumatology Society of the Community of Madrid (SORCOM), was carried out to evaluate the implementation of a previously developed checklist for the assessment of patients with SpA. The study began in February 2016 and ended in September 2018. It was approved by all the ethics committees of the participating hospitals and was conducted in accordance with Best Clinical Practice and the current version of the revised Declaration of Helsinki.
Development and characteristics of the assessment checklistThe design and characteristics of the assessment checklist for patients with axSpA and PsA in daily practice (ONLY TOOLS project) have been described earlier.8 In short, this was a qualitative study that included: (1) a nominal group of 18 experts in SpA; (2) literature reviews, and (3) two focus groups, one with rheumatologists and another with SpA patients. The checklist was created from all of this, and includes different variables for the assessment of these patients in daily practice (and their frequency).
Práctica Madrid studyThe checklist was implemented in the Práctica Madrid project, a prospective observational study that is also described in a previous article (pending publication). Its objective was to analyse the feasibility and changes in the collection of variables in medical records after implementing the checklist in daily practice. The analysis of possible changes in the collection of variables requires two groups of patients, before and after the implementation of the tool, in order to compare the changes produced with it.
In summary, we selected a convenience sample of 6 hospitals in the Community of Madrid, with different characteristics in terms of resources, population served and rheumatology department characteristics. In each hospital, a sample of rheumatologists was invited to participate, covering all possible professional category profiles (resident, assistant, head of section and head of department), age and sex. The selected centres cover a wide spectrum of infrastructure, size, pre- and post-graduate teaching, and other characteristics. This variability allows us to assume that the results could be representative at a national level.
We then selected consecutive patients with SpA (50% axSpA, 50% PsA) who had been seen in the rheumatology departments at least once in the year prior to the start date of the study (February 2016). Clinical records were reviewed and an electronic data collection notebook (DCN) was completed prior to implementation. The checklist was then used by the rheumatologist in daily practice for at least one week, after which its feasibility and practicality were assessed by means of a questionnaire addressed to the professionals responsible for completing it, which included variables such as checklist execution time, simplicity, clarity and usefulness. Each of these sections was scored from 1 to 10 (1=very little to 10=very/high).
Six months later, the medical records of another group of consecutive patients (different from those of the pre-implementation phase) were reviewed again and the post-implementation electronic DCN was completed. These DCNs include, among others, checklist variables such as: (1) age, sex, physical activity or smoking habit; (2) similar disease duration or family history; (3) comorbidities such as obesity or depression; (4) biomarkers such as rheumatoid factor or HLA-B27; (5) physical examination, including enthesitis or dactylitis; (6)variables of activity and function; (7) laboratory tests; (8) imaging studies, and (9) treatments.
Statistical analysisA descriptive analysis of baseline characteristics and changes in the recording of variables was performed for patients with axSpA and PsA separately. Quantitative variables were described using median and interquartile range, and qualitative variables were described in frequencies and percentages. For comparisons, we used the Student's t- or Mann–Whitney U test for continuous variables and χ2 test for categorical variables. The analyses were performed using Stata 12 statistical software (Stata Corporation, College Station, TX, USA).
ResultsSix hospitals and 11 rheumatologists participated. A total of 83 and 68 medical histories pre and post implementation of the checklist were reviewed. The patient characteristics of both samples were similar, with a median age at diagnosis of 43 and 40 years, respectively, and an interquartile range (p25–75) of 33–51 and 32–48, respectively. About half of the patients were male. The proportion of patients with axSpA and PsA was comparable (40.2% and 44.8% pre-implementation, 47.6% and 47.8% post-implementation). Eight percent of the patients with axSpA also had peripheral involvement, and none of the patients with PsA had axial involvement.
At baseline (Tables 1 and 2) we highlight that, except for some variables such as nail involvement or uveitis, the general trend is towards a lower percentage of recording in patients with PsA compared to those with axSpA.
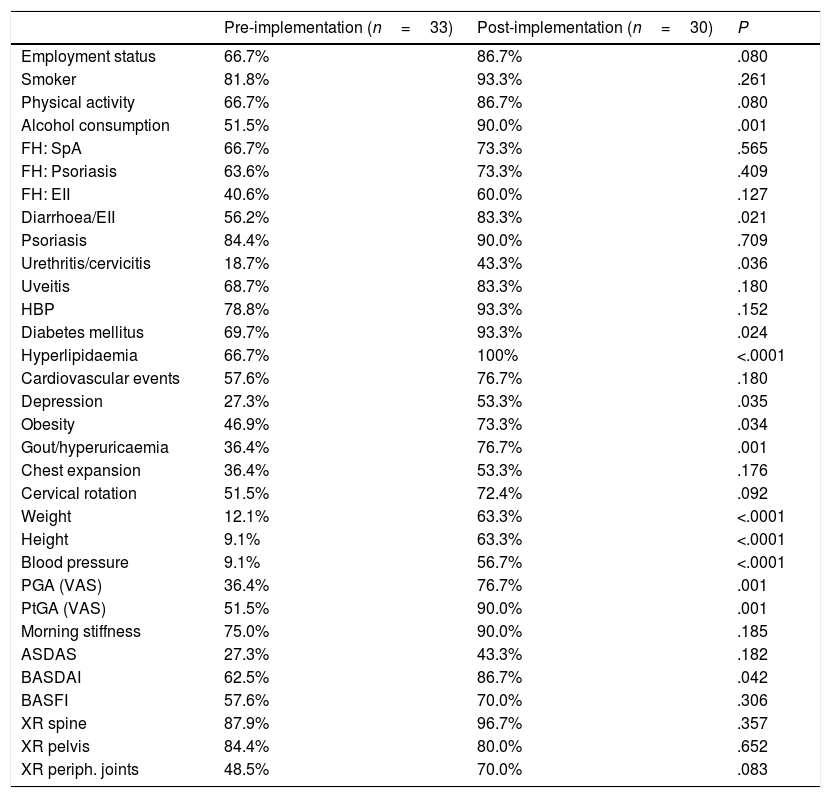
Changes in the recording of variables after implementation of the assessment checklist for patients with axial spondyloarthritis.
| Pre-implementation (n=33) | Post-implementation (n=30) | P | |
|---|---|---|---|
| Employment status | 66.7% | 86.7% | .080 |
| Smoker | 81.8% | 93.3% | .261 |
| Physical activity | 66.7% | 86.7% | .080 |
| Alcohol consumption | 51.5% | 90.0% | .001 |
| FH: SpA | 66.7% | 73.3% | .565 |
| FH: Psoriasis | 63.6% | 73.3% | .409 |
| FH: EII | 40.6% | 60.0% | .127 |
| Diarrhoea/EII | 56.2% | 83.3% | .021 |
| Psoriasis | 84.4% | 90.0% | .709 |
| Urethritis/cervicitis | 18.7% | 43.3% | .036 |
| Uveitis | 68.7% | 83.3% | .180 |
| HBP | 78.8% | 93.3% | .152 |
| Diabetes mellitus | 69.7% | 93.3% | .024 |
| Hyperlipidaemia | 66.7% | 100% | <.0001 |
| Cardiovascular events | 57.6% | 76.7% | .180 |
| Depression | 27.3% | 53.3% | .035 |
| Obesity | 46.9% | 73.3% | .034 |
| Gout/hyperuricaemia | 36.4% | 76.7% | .001 |
| Chest expansion | 36.4% | 53.3% | .176 |
| Cervical rotation | 51.5% | 72.4% | .092 |
| Weight | 12.1% | 63.3% | <.0001 |
| Height | 9.1% | 63.3% | <.0001 |
| Blood pressure | 9.1% | 56.7% | <.0001 |
| PGA (VAS) | 36.4% | 76.7% | .001 |
| PtGA (VAS) | 51.5% | 90.0% | .001 |
| Morning stiffness | 75.0% | 90.0% | .185 |
| ASDAS | 27.3% | 43.3% | .182 |
| BASDAI | 62.5% | 86.7% | .042 |
| BASFI | 57.6% | 70.0% | .306 |
| XR spine | 87.9% | 96.7% | .357 |
| XR pelvis | 84.4% | 80.0% | .652 |
| XR periph. joints | 48.5% | 70.0% | .083 |
ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; IBD: inflammatory bowel disease; SpA: spondyloarthritis; VAS: visual analogue scale; FH: family history; HBP: high blood pressure; periph.: peripheral; XR: plain X-ray; PGA: physician global assessment; PtGA: patient global assessment.
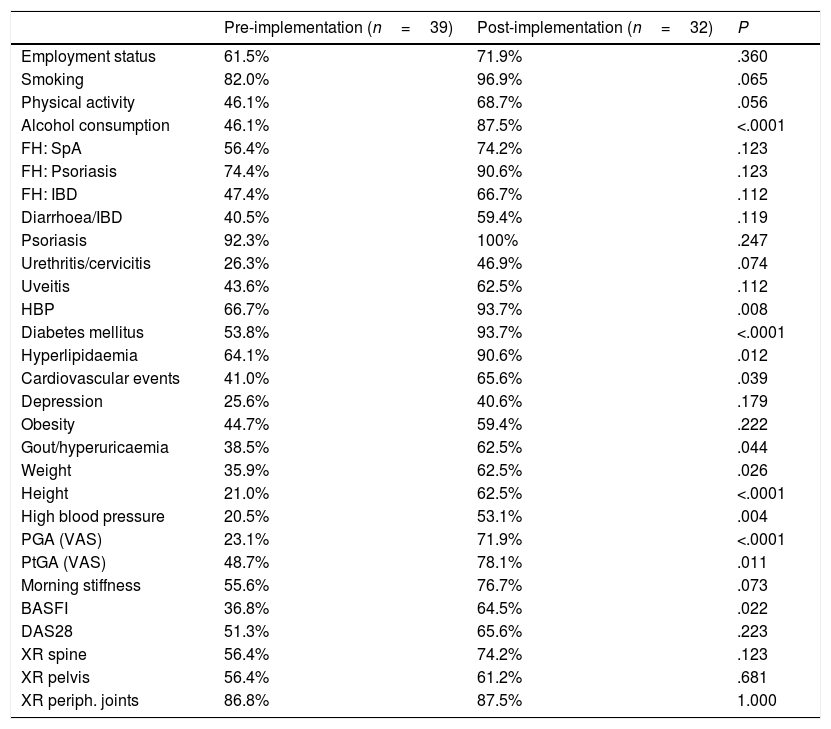
Changes in the recording of variables after implementation of the assessment checklist for patients with psoriatic arthritis.
| Pre-implementation (n=39) | Post-implementation (n=32) | P | |
|---|---|---|---|
| Employment status | 61.5% | 71.9% | .360 |
| Smoking | 82.0% | 96.9% | .065 |
| Physical activity | 46.1% | 68.7% | .056 |
| Alcohol consumption | 46.1% | 87.5% | <.0001 |
| FH: SpA | 56.4% | 74.2% | .123 |
| FH: Psoriasis | 74.4% | 90.6% | .123 |
| FH: IBD | 47.4% | 66.7% | .112 |
| Diarrhoea/IBD | 40.5% | 59.4% | .119 |
| Psoriasis | 92.3% | 100% | .247 |
| Urethritis/cervicitis | 26.3% | 46.9% | .074 |
| Uveitis | 43.6% | 62.5% | .112 |
| HBP | 66.7% | 93.7% | .008 |
| Diabetes mellitus | 53.8% | 93.7% | <.0001 |
| Hyperlipidaemia | 64.1% | 90.6% | .012 |
| Cardiovascular events | 41.0% | 65.6% | .039 |
| Depression | 25.6% | 40.6% | .179 |
| Obesity | 44.7% | 59.4% | .222 |
| Gout/hyperuricaemia | 38.5% | 62.5% | .044 |
| Weight | 35.9% | 62.5% | .026 |
| Height | 21.0% | 62.5% | <.0001 |
| High blood pressure | 20.5% | 53.1% | .004 |
| PGA (VAS) | 23.1% | 71.9% | <.0001 |
| PtGA (VAS) | 48.7% | 78.1% | .011 |
| Morning stiffness | 55.6% | 76.7% | .073 |
| BASFI | 36.8% | 64.5% | .022 |
| DAS28 | 51.3% | 65.6% | .223 |
| XR spine | 56.4% | 74.2% | .123 |
| XR pelvis | 56.4% | 61.2% | .681 |
| XR periph. joints | 86.8% | 87.5% | 1.000 |
ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; IBD: inflammatory bowel disease; SpA: spondyloarthritis; VAS: visual analogue scale; FH: family history; HBP: high blood pressure; periph.: peripheral; XR: plain X-ray; PGA: physician global assessment; PtGA: patient global assessment.
On the other hand, in general, implementation increased the recording of almost all variables in all patients, regardless of the SpA subtype. Specifically, in patients with axSpA (Table 1) there was a significant increase in the recording of alcohol consumption in the clinical history (51% vs 90%), as well as some extra-articular manifestations such as diarrhoea or inflammatory bowel disease (IBD) and urethritis, comorbidities such as diabetes mellitus (69% vs 93%), hyperlipidaemia (66% vs 100%), depression, obesity or gout/hyperuricaemia, and the following physical examination data: weight (12% vs 63%), height (9% vs 63%) and blood pressure (9% vs 56%). The recording of other disease-related parameters also increased, such as physician global assessment (PGV) (36% vs 76%) and patient global assessment (PGV) (51% vs 90%), and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (62% vs 86%).
In the case of PsA (Table 2), the parameters whose recording in medical records increased significantly were alcohol consumption (46% vs 87%), comorbidities such as high blood pressure (HBP) (66% vs 93%), diabetes mellitus (53% vs 93%), and hyperlipidaemia (64% vs 90%), cardiovascular disease (42% vs 65%) and gout/hyperuricaemia (38% vs 62%), and almost all physical examination parameters, such as chest expansion (23% vs 50%), cervical rotation (20% vs 53%), weight (35% vs 62%), height (21% vs 62%) and blood pressure (23% vs 71%). The same was true of the PGA (23% vs 71%), the PtGA (48% vs 78%) and the Bath Ankylosing Spondylitis Functional Index (BASFI) (36% vs 64%).
Comparatively, as at the baseline, the trend is towards a higher percentage of collection in axSpA than in PsA (Tables 1 and 2). On the other hand, we also note that post-implementation, in both groups, there are variables that continue to be collected in nearly 50% of the records, or even less, such as the presence of depression, chest expansion or Ankylosing Spondylitis Disease Activity Score (ASDAS).
DiscussionThe checklist developed for the ONLY TOOLS project was considered in this sub-analysis of the Práctica Madrid study. The results obtained have shown that the implementation of a specific checklist improves, in general terms, the assessment of patients with axSpA as well as with PsA. Furthermore, it has been observed that in the PsA subgroup, pre- and post-implementation of the checklist, the percentage of collection of relevant variables is clearly insufficient. Finally, and despite the increase observed after the implementation of this action framework, the collection of some variables continues to be low in both groups. Although in a study designed for a different purpose (adherence to a treat-to-target strategy protocol, with patients with SpA and RA), Lesuis et al.10 found that after the implementation of an assessment protocol they went from collecting 9% of cases to 20%. Although the trend is upwards, these figures are much lower than those collected in this study.
Since the EmAR II study, conducted in 2009–2010, which found poor recording in the daily practice of assessment of patients with SpA, many national and international recommendations have been published and various projects have been developed to improve the management of patients with SpA.1,11–14 The data collected at the baseline in the Práctica Madrid project in 2018 show an increase in the collection of variables in the assessment of patients with axSpA and PsA, suggesting a positive effect of all the activities discussed, which clearly continues to improve after the implementation of the checklist, regardless of the subgroup.
Despite the improvement mentioned in the collection of variables, there continues to be suboptimal evaluation of processes of high prevalence or impact, such as depression,4 or of activity and function parameters, such as chest expansion, ASDAS or Disease Activity Score 28 (DAS28). In the current framework of SpAs, where referral is the main objective and where decisions are made through holistic and patient-centred assessment, the emphasis should continue to be on improving the assessment of patients with axSpA especially in PsA, despite the good results obtained.
This study is not free of limitations, mainly related to the small sample size and the representativeness of the sample. It is evident that the study of 83 medical records before the implementation of the checklist and 68 after its implementation may seem, at first glance, insufficient to detect an effect. However, despite this reduced number, significant differences have been found in various variables, indicating that the effect is large enough to be detected in small samples and that larger samples would predictably ratify the results observed. On the other hand, and in order to avoid overestimation of the effect, in the post-implementation phase of the tool, different patients to those in the first phase were selected, in order to avoid an excess of variables in previously selected patients. Secondly, the design used, with the selection of centres with different characteristics and resources, aims to ensure the representativeness of the sample. Despite these considerations, it is clear that this analysis could be a first point of departure for the proposed objective that would justify conducting a study with a greater number of patients and centres.
FundingThis project was conducted with the collaboration of the Rheumatology Society of the Community of Madrid (SORCOM).
Conflict of interestsE.L. has received funding for research projects from Roche, MSD, Pfizer, Abbvie, BMS, UCB, Actelion, Celgene, Grunenthal and Sanofi. E.R.A. has received funding for research projects and courses from GSK, Abbvie, MSD and Celgene. E.R. has received funding for courses from Pfizer, Abbvie, Novartis, Roche, Lilly, Janssen and Asacpharma. The remaining authors have no conflict of interests to declare.
To Dr M. Jesús García de Yébenes for her methodological and statistical advice.
Please cite this article as: Almodovar R, Joven B, Rodríguez Almaraz E, Melchor S, Rabadán E, Villaverde V, et al. Resultados comparativos de la implementación en práctica diaria de un checklist de evaluación para pacientes con espondiloartritis axial y artritis psoriásica. Reumatol Clin. 2021;17:392–396.