Septic pyogenic arthritis of the acromioclavicular joint is a rare entity that occurs in immunosuppressed patients or those with discontinuity of defense barriers. There are only 15 cases described in the literature. The diagnosis is based on clinical features and the isolation of a microorganism in synovial fluid or blood cultures. The evidence of arthritis by imaging (MRI, ultrasound or scintigraphy) may be useful. Antibiotic treatment is the same as in septic arthritis in other locations. Staphylococcus aureus is the microorganism most frequently isolated.
Our objective was to describe the clinical features, treatment and outcome of patients diagnosed with septic arthritis of the acromioclavicular joint at a Rheumatology Department. We developed a study with a retrospective design (1989–2012). The medical records of patients with septic arthritis were reviewed (101 patients). Those involving the acromioclavicular joint were selected (6 patients; 6%).
La artritis séptica de la articulación acromioclavicular (ACV) es una entidad poco frecuente que se presenta en pacientes inmunosuprimidos o con discontinuidad de las barreras de defensa. En la literatura únicamente se han descrito 15 casos hasta la fecha. El diagnóstico se basa en la presencia de clínica compatible junto al aislamiento del germen en la articulación o en los hemocultivos. Las técnicas de imagen (resonancia magnética, ecografía o gammagrafía) pueden ser útiles en la localización del proceso. El tratamiento antibiótico es el mismo que en la artritis séptica de otra localización y Staphylococcus aureus es también el microorganismo aislado con más frecuencia.
Se describen las características clínicas, el tratamiento y la evolución de los pacientes diagnosticados de artritis séptica de la articulación ACV en un servicio de Reumatología, mediante estudio retrospectivo de revisión de historias clínicas de los pacientes atendidos por artritis séptica en dicha unidad (101 pacientes) en el periodo de 1989-2012. Seis enfermos (6%) tuvieron infección en la articulación ACV con confirmación microbiológica.
Often, trauma and inflammatory diseases affect the acromioclavicular (ACV) joint. However, septic arthritis is rarely seen in this localization. In Anglo-Saxon literature reports only 15 cases described to date in detail (Table 1).1–11 In most cases it occurs in patients with risk factors such as immunosuppression. Diagnosis is based on clinical data and isolation of the etiologic microorganism in1 microbiological cultures, although imaging tests, such as magnetic resonance, ultrasound or scintigraphy, may contribute to the demonstration of arthritis. Here, we describe the clinical, laboratory, imaging and therapeutic data in a series of 6 patients with septic arthritis of the ACV joint, diagnosed in a university hospital center.
Summary of the Clinical, Epidemiological and Microbiological Characteristics of the Cases Described in the Biomedical Literature.
| Gender and age | Causal agent | Joint location | Risk factors | Likely entry gateway | Hematogenous spread | Fever | Diagnostic interval (days) | Imaging tests showing ACV joint arthritis | Antibiotic treatment | Evolution |
| Male, 68 years2 | Streptococcus B | Right Acromioclavicular | DiabetesVenous insufficiency ulcers | Ulcers | Yes | Yes | 1. | NMR | Ampicillin iv | Healed |
| Male, 48 years3 | Streptococcus viridans | Right Acromioclavicular | No | Unknown | No | Yes | 2. | Radiography | Penicillin iv | DebridementHealed |
| Male, 42 years4 | Staphylococcus aureus | Left acromioclavicular | No | Unknown | Yes | Yes | 2. | RadiographyNMR | Iv flucloxacillinFusidic acid vo | Healed |
| Male, 63 years5 | Staphylococcus aureus | Left acromioclavicular | No | Unknown | No | Yes | 2. | RadiographyNMR | Cephalosporins iv | DebridementHealed |
| Female, 65 years6 | Haemophilus parainfluenzae | Left acromioclavicular | No | Unknown | No | No | 7. | RadiographyNMR | Levofloxacin vo | DebridementHealed |
| Male, 63 years7 | Staphylococcus aureus | Unilateral acromioclavicular | No | Unknown | Yes | Yes | 5. | RadiographyNMR | Iv oxacillin | DebridementHealed |
| Male, 72 years1 | Staphylococcus aureus | Left acromioclavicularLeft wrist | No | Endocarditis | Yes | Yes | 2. | – | Oxacillin andiv and gentamycin iv | Death |
| Male, 55 years1 | Staphylococcus aureus | Right AcromioclavicularPsoas abscess | DiabetesGout | Unknown | Yes | Yes | 2. | NMR | Iv oxacillin and ciprofloxacin iv | Healed |
| Male, 38 years1 | Staphylococcus aureus | Right AcromioclavicularLeft sternoclavicular | HCVHBV | Use of IV drugs | No | Yes | 28 | RadiographyCT | Iv iv ofloxacin and rifampicin | DebridementHealed |
| Male, 62 years1 | Staphylococcus aureus | Right Acromioclavicular | No | Glucocorticoid infiltration | Yes | Yes | 2. | UltrasoundNMR | Ofloxacin and cloxacillin iv iv | Healed |
| Female, 55 years8 | Streptococcus pneumoniae | Left acromioclavicular | Chemotherapy multiple myelomaRenal | Porth-a-cath | Yes | Yes | 3. | NMR | Ceftriaxone iv | DebridementHealed |
| Female, 79 years8 | Group B Streptococcus | Right Acromioclavicular | Not | Unknown | No | Yes | 4. | – | Ceftriaxone iv | Healed |
| Male, 44 years9 | Staphylococcus aureus | Left acromioclavicular | Diabetes | Unknown | Yes | Yes | 6. | UltrasoundScintigraphy with labeled WBCs | Iv iv cloxacillin and gentamicin | Healed |
| Male, 25 years10 | Staphylococcus aureus | Right Acromioclavicular | AIDS | Unknown | No | No | – | Radiography | Ciprofloxacin vo | Healed |
| Male, 17 years12 | Ochrobactrum anthropi | Right Acromioclavicular | No | Trauma | No | No | 7. | NMR | Ciprofloxacin and co-trimoxazole vo vo | DebridementHealed |
The study was a retrospective review (1989–2012) of the medical records of patients with septic arthritis found in the database of diagnostic coding of the Rheumatology department of a university hospital, with a reference population of 800000. The Rheumatology department takes care of the diagnosis and treatment of the vast majority of non-prosthetic septic arthritis. Thus, in this period, 101 patients were diagnosed with pyogenic septic arthritis, 6 of whom (6.1%) had ACV joint involvement. All patients had a microbiological germ isolation in joint fluid obtained by arthrocentesis of the ACV joint. The following variables were collected: epidemiological, clinical manifestations, risk factors, imaging, treatment received and laboratory tests such as complete blood count (Coulter), erythrocyte sedimentation rate, C reactive protein (CRP by nephelometry), biochemistry, blood and joint fluid culture.
ResultsIn this period 101 patients with pyogenic septic arthritis were diagnosed at the Rheumatology department. Six patients had ACV septic arthritis (6%): five male and one female, mean age at diagnosis of 51.2 years (range 46–73). All patients had some risk factor and the most common was chronic renal failure, present in 4 patients (66.7%). In all cases there was a site of entry and in 2 patients it was an intravascular device (33.3%). One patient had a history of gout and ankylosing spondylitis, while the rest did not have a prior arthropathy (Table 2). The median time from onset of symptoms to diagnosis was 6 days (range 2–10).
Summary of Clinical, Epidemiological, Microbiological Characteristics of Patients in the Series.
| Gender and age | Causal agent | Joint location | Risk factors | Likely entry gateway | Hematogenous spread | Fever | Diagnostic interval (days) | Imaging tests showing ACV joint arthritis | Antibiotic treatment and duration | Evolution |
| Male, 73 years | Staphylococcus aureus | Right Acromioclavicular | CirrhosisChronic renal failure | Articular infiltration | Yes | No | 7 | Ultrasound | Intravenous CloxacillinCiprofloxacin oral(6 weeks) | Surgical debridementHealed |
| Female, 46 years | Staphylococcus aureus | Right Acromioclavicular | Disseminated breast neoplasiaChemotherapy | Porth to cath | Yes | Yes | 7 | Ultrasound | Intravenous Cloxacillin | Death 9th day |
| Male, 72 years | Staphylococcus aureus | Left acromioclavicularKneesRight elbow | Chronic renal failure | Skin lesions | Yes | Yes | 10 | UltrasoundScan | Intravenous CloxacillinCiprofloxacin oral(6 weeks) | Admission to ICUHealed |
| Male, 52 years | Streptococcus pneumoniae | AcromioclavicularRight hip | Diabetes mellitus | Respiratory | Yes | Yes | 4 | Scan | Penicillin G Sodium intravenousOral amoxicillin(8 weeks) | Healed |
| Male, 53 years | Streptococcus agalactiae | AcromioclavicularRight KneeLeft hip | Chronic renal failure | Skin lesion with cellulitis and abscess | Yes | Yes | 2 | Scan | Penicillin G Sodium intravenousOral amoxicillin(8 weeks) | Knee DebridementGirdlestone hipHealed |
| Male, 71 years | Staphylococcus aureus | Right Acromioclavicular | Chronic renal failureAlcoholism | Peripherally and phlebitis | Yes | Yes | 5 | None due to heart failure and hemodynamic instability | Intravenous Cloxacillin | Good evolution of sepsisDeath by hematoma of rectus sheath |
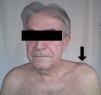
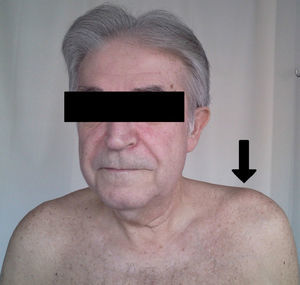
All patients had swelling and localized pain in the affected joint and pain on ACV abduction and flexion of the shoulder in extreme movements (Fig. 1). Four patients had fever as the initial manifestation, all showing leukocytosis and elevated acute phase reactants: the average C-reactive protein was 105±22U/L and an erythrocyte sedimentation rate of 85±19mm/h. Arthritis was monoarticular in 3 patients and polyarticular in 3. Bilateral involvement occurred in 2 cases.
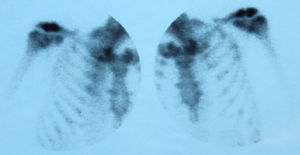
To confirm the clinical diagnosis of ACV arthritis, ultrasonography was performed in 3 cases, finding abscessed collections in soft tissues around the ACV joint. In 3 cases, scintigraphy was especially useful in patients with polyarticular affection (Fig. 2). We were unable to perform imaging in one of the patients due to clinical instability. Plain radiographs showed no erosions in any of the cases. MRI was not performed in any of them.
ACV joint arthrocentesis was performed in all cases, with isolation of the causal pathogen through a joint fluid culture. The most frequently isolated organism was Staphylococcus aureus (S. aureus) (4 patients, 6.7%). In the other 2 cases the isolated organism was a streptococcus (one Streptococcus pneumoniae, one Streptococcus agalactiae). All patients had positive blood cultures due to hematogenous spread.
All patients received intravenous antibiotic treatment for at least 3 weeks and oral antibiotics to complete a minimum of six weeks, with the exception of patients who died early. Patients initially received empirical antibiotic therapy, which subsequently was adapted to the result of the antibiogram. Antibiotic treatment is detailed in Table 2.
Articular lavage were performed in 2 patients and another required surgical drainage of the ACV joint. Another patient, the most severely immunosuppressed, died 9 days after the diagnosis of septic shock, despite having monoarticular involvement. Another patient died nine days from hypovolemic shock caused by a hematoma of rectus abdominis sheath, in the context of anticoagulation for acute myocardial infarction. The cases with polyarticular disease presented a more torpid evolution, but eventually recovered completely. There was evidence of greater diagnostic delay in monoarticular cases.
DiscussionBacterial infectious arthritis is monoarticular in 85% of cases and most commonly affects the knee. In the absence of trauma or joint manipulation, hematogenous spread is the most common cause. For this reason, the large joints, which are more vascularized, have more risk of septic arthritis.12
Septic arthritis of the ACV joint is a rare entity, although rapidly destructive. It usually occurs in immunosuppressed patients or those with a discontinuity of defense barriers, but even in this group of patients it is unusual. However, the diagnosis cannot be excluded in healthy patients.1 Its low incidence explains the limited number of patients seen in a single hospital over a period of 22 years. For this reason, the largest global series includes 4 patients and we have found a total of 15 described in the literature, most of them reported as isolated cases.1–11 We did not include cases in which there was a germ isolated from blood cultures or cultures of joint fluid. Shoulder arthritis in large series of septic arthritis is common, around 5%–10%. These sets differ sternoclavicular arthritis, but ACV arthritis is recognized in many cases along with the glenohumeral joint, so its true incidence cannot be known.12–21 The diagnostic coding system used in our hospital allowed us to differentiate glenohumeral septic arthritis to that located on the ACV joint, so we were able to justify the 6% incidence described in the present series. Possibly, in many published series of septic arthritis, ACV infectious arthritis is included in those located on the shoulder.
The demographic characteristics of our series are similar to those described by Bossert et al.1 The higher percentage of males is noteworthy. The mean age at diagnosis was also 50–60 years.
Typically, its clinical presentation is characterized by swelling and pain over the joint, but in many cases the shoulder range of motion is preserved, although it may be painful in extreme movements. Thorough clinical inspection is essential for diagnosis. Its course is acute and early diagnosis occurs often. They are serious infections and hematogenous dissemination and polyarticular involvement are poor prognostic factors. We should not forget this location in the presence of septic arthritis from other locations, as it probably often goes unnoticed in the course of septic arthritis in more striking locations. The polytropic incidence of septic arthritis in our series was 29%, and yet, in the case of ACV, half of them had more than one affected joint.22
Arthrocentesis should be performed early in the disease and lead to a definitive diagnosis. It is essential to perform blood cultures and joint fluid culture. S. aureus is the most frequently isolated germ, followed by various types of streptococci. These results agree with those of other published cases and the studies of septic arthritis of other joints.1–11 There is a high percentage of positive blood cultures, indicating that there is usually hematogenous spread. Notably, in this series, it was present in all cases. Therefore, it is important to interrogate the patient about a possible gateway and consider the concomitant existence of infective endocarditis. It is noteworthy that in this series there were no patients addicted to intravenous drugs, but our hospital is a referral center for patients suffering from human immunodeficiency virus infection.
Antibiotic treatment is the same as in septic arthritis in another location and it is recommended that it initially be given intravenously. It is advised that the duration of antibiotic last not less than 4 weeks. Sometimes medical treatment by itself may be ineffective and it may be necessary to conduct joint lavages or surgical drainage.12–21 Regarding imaging tests, simple X rays must be carried out but changes can be observed late, such as erosions. Ultrasound and MRI allow earlier diagnosis and assess the local extension of the infection. Ultrasound will show an increase in joint space with a loosening of the capsule and it will likewise allow us to make guided punctures to obtain joint fluid. Magnetic resonance has greater definition of tissue, it detects earlier damage, is sensitive and specific, and is not operator-dependent. In patients with polyarticular or paucisymptomatic, scintigraphy may help with the diagnosis.23–25 The main limitations of this study include its retrospective nature and the limited number of patients, as well as the low incidence of this disease.
In conclusion, septic arthritis of the ACV joint is a rare entity, although it may be underdiagnosed. It generally affects immunosuppressed individuals, but cannot be excluded in healthy patients. Since this is a small joint, there is a high risk of fast joint destruction, so early diagnosis is crucial. Although arthrocentesis remains the diagnostic test of choice, the procedure can be difficult due to the characteristics of the joint. Therefore, imaging tests such as ultrasound or magnetic resonance imaging may be useful. Hematogenous spread is practically constant, as may be the existence of a entry gateway. Polyarticular involvement and immunosuppression are poor prognostic factors. Antibiotic treatment should be started early.
Key Points- -
Rare, but probably underdiagnosed.
- -
Immunosuppression or discontinuity of defense barriers is risk factors.
- -
It appears as an acute monoarthritis, but must be sought in septic polyarthritis.
- -
The definitive diagnosis is provided by the isolation of the organism in joint fluid.
- -
Staphylococcus aureus is the most frequently isolated germ.
- -
Hematogenous spread is rare.
- -
Hematogenous dissemination and polyarticular involvement are poor prognostic factors.
The authors declare that experiments have not been performed on humans or animals.
Data ConfidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to Privacy and Informed ConsentThe authors have obtained the informed consent of patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Martínez-Morillo M, Mateo Soria L, Riveros Frutos A, Tejera Segura B, Holgado Pérez S, Olivé Marqués A. Artritis séptica de la articulación acromioclavicular: una localización atípica. Reumatol Clin. 2014;10:37–42.