The elbow patients herein discussed feature common soft tissue conditions such as tennis elbow, golfers’ elbow and olecranon bursitis. Relevant anatomical structures for these conditions can easily be identified and demonstrated by cross examination by instructors and participants. Patients usually present rotator cuff tendinopathy, frozen shoulder, axillary neuropathy and suprascapular neuropathy. The structures involved in tendinopathy and frozen shoulder can be easily identified and demonstrated under normal conditions. The axillary and the suprascapular nerves have surface landmarks but cannot be palpated. In neuropathy however, physical findings in both neuropathies are pathognomonic and will be discussed.
Se consideran ciertas patologías de los tejidos blandos del codo y del hombro. El codo de tenista, el codo de golfista y la bursitis olecraniana afectan estructuras anatómicas fácilmente identificables y demostrables en el examen cruzado de instructores y participantes. Los temas de hombro incluyen la tendinopatía del manguito rotador, el hombro congelado, la neuropatía del nervio axilar y la neuropatía del nervio supraescapular. En las tendinopatías y el hombro congelado la anatomía relevante es fácilmente identificable y demostrable. No así en las neuropatías que carecen de reparos anatómicos aunque son fácilmente demostrables por los déficits que causan en el examen de pacientes afectados. Este conjunto de estructuras se analiza desde un punto de vista anatómico general.
The elbow is a seemingly simple joint. It has the appearance of a plain hinge between 3 bones, the humerus above and the ulna and radius below (Fig. 1). The main action of the humeroulnar joint is flexion and extension of the elbow. The ulna is also adducted (brought closer to the body) in supination and abducted in pronation. The humeroradial or capituloradial joint also participates in flexion and extension of the elbow. However, a glance at both ends of the forearm bones indicates that the radius is specialized to rotate around a fixed point proximally and with a greater arc of motion distally (Fig. 2a and b). The size of their ends tells something about the function of ulna and radius. Ulna is thick proximally to provide stability to the elbow hinge while radius is thick distally to provide a stable ellipsoid joint to the wrist. All of the involved joints, the humeroulnar, the humeroradial, the proximal radioulnar and the distal radioulnar joints work in coordination to shorten and extend the upper limb and, at the same time, to supinate and pronate the forearm. This frees the hand to operate almost instantly in opposite planes. The maximal congruency between the humerus and the ulna in extension is attained at an angle open to the side between 5° and 15° known as the “carrying angle of the elbow” because it prevents the knee from kicking a weight carried on the side.
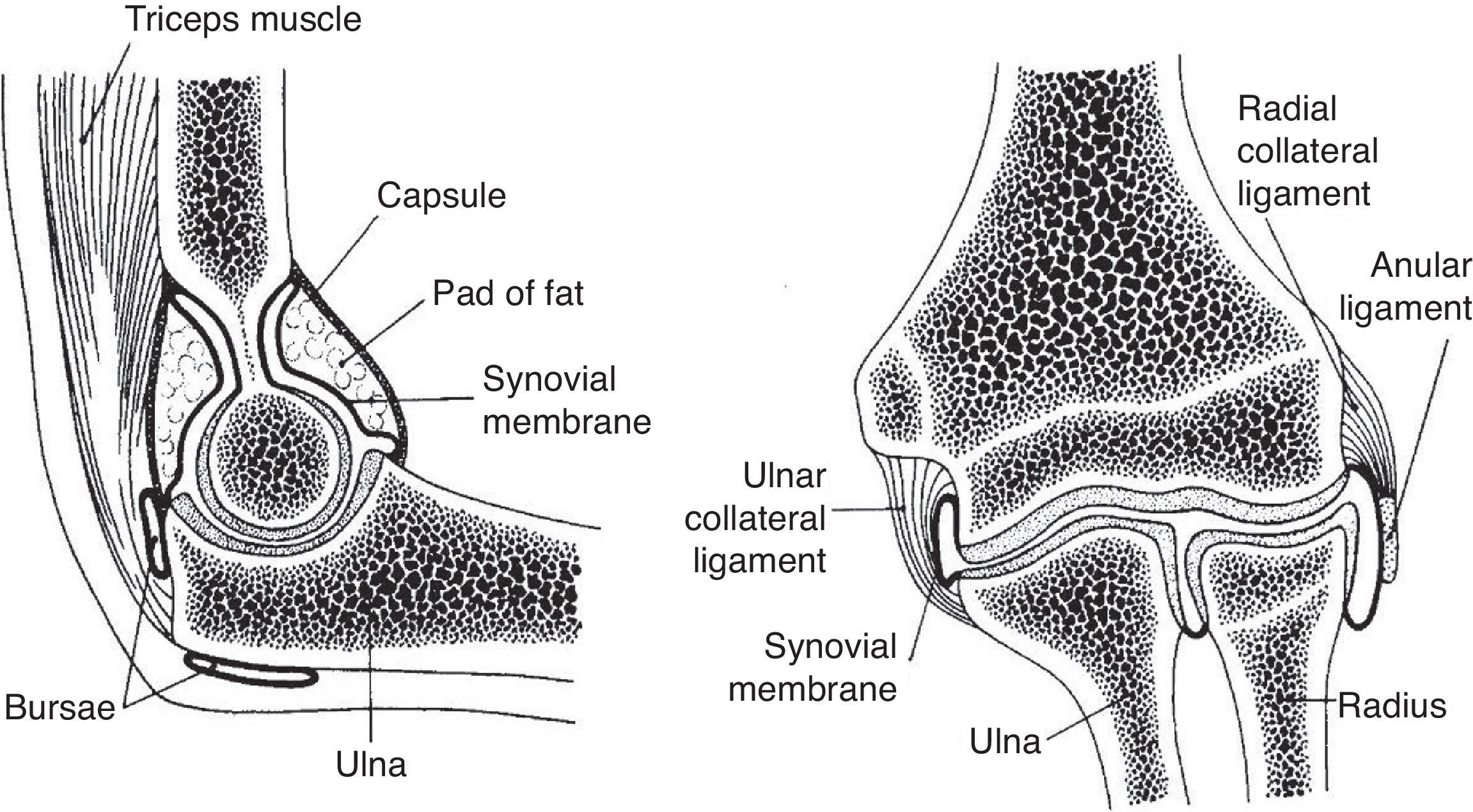
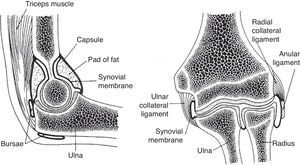
A frontal and a sagittal section of the elbow are shown. In the left panel two bursae are seen. One, the olecranon bursa, is a subcutaneous structure. The other is a bursa placed deep to the distal triceps tendon. The synovial cavity is seen posteriorly at the olecranon fossa and anteriorly at the coronoid fossa. In both the synovial cavity is separated from the capsule by a fat pad. In the right panel the widely interconnecting humeroradial, humeroulnar and radioulnar portions of the elbow joint are shown.
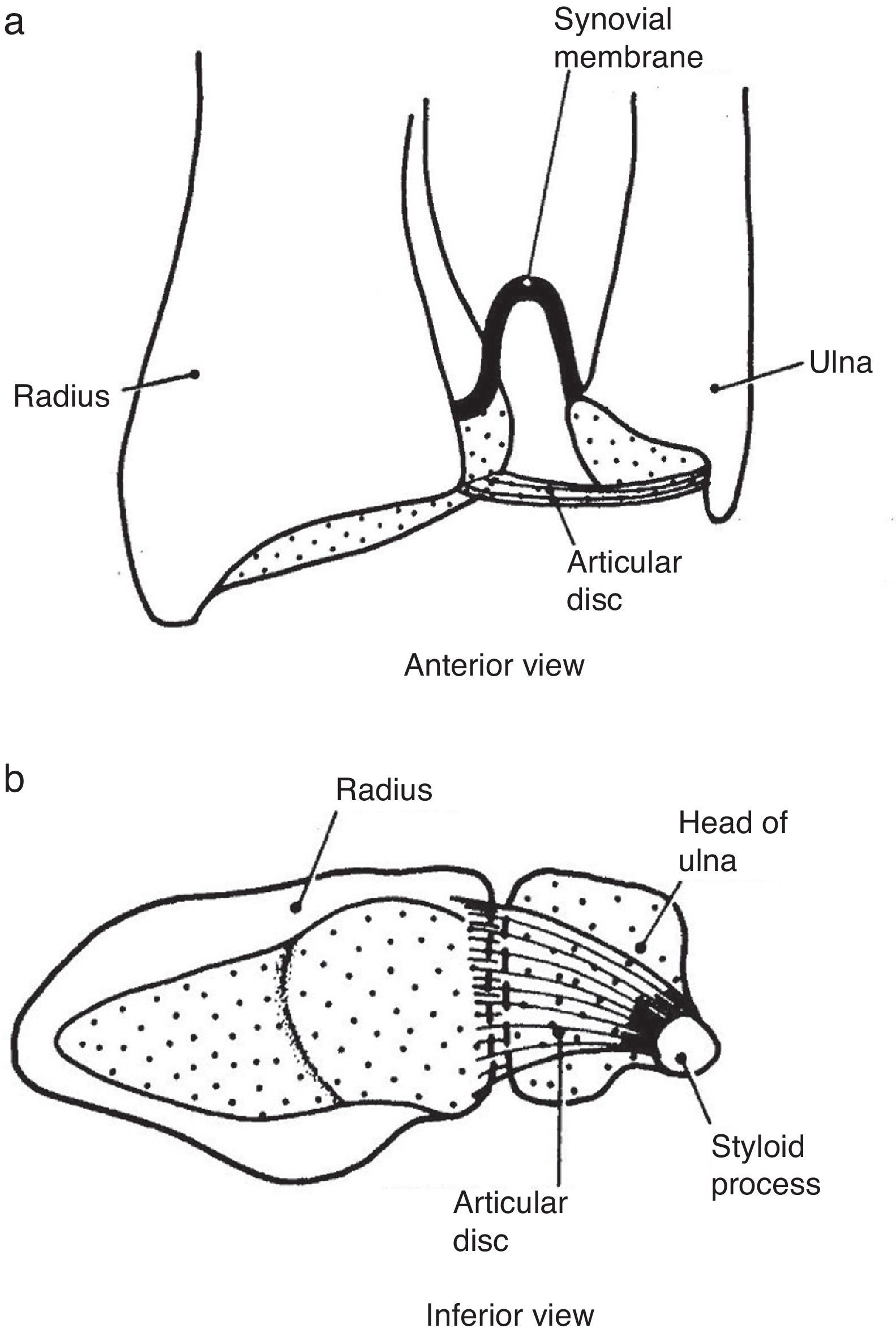
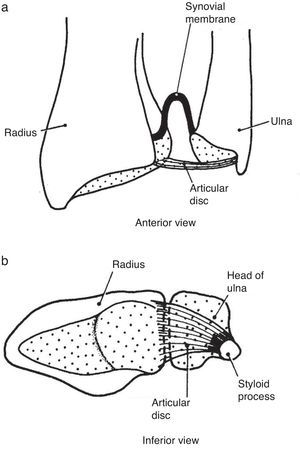
(a) The distal radioulnar joint is shown. The articular disc is also known by clinicians as triangular cartilage. A synovial fold, or sacciform recess, is seen proximal to the articular surfaces. (b) An “on end” view of the distal radioulnar joint. The articular disc is attached to the ulnar styloid by its apex and by its base to the radius. The disc slides over the ulnar end during pronation and supination of the forearm.
Patient 1. Tennis elbow “A 67 year old man with pain in the lateral aspect of his right elbow; pain is increased by resisted wrist extension”.
Patient 2. Golfers elbow “A 53 year old woman with pain in her medial elbow; pain is increased by resisted wrist flexion”
Patients 1 and 2 are typical examples of two conditions that result from repetitive stress at a tendon origin, “tennis elbow” and “golfer's elbow”. Tennis elbow or lateral epicondylitis is characterized by a triad of clinical findings: lateral elbow pain, epicondylar tenderness and pain upon resisted extension of the wrist.1 Golfer's elbow or medial epicondylitis is a mirror image of tennis elbow: medial elbow pain, medial epicondylar tenderness and pain upon resisted flexion of the wrist.2,3 Therefore, these two cases bring us to the origin of 2 groups of forearm muscles, the wrist extensors and the wrist flexors. In addition to discussing these muscles, certain relevant aspects of the radial and ulnar nerves will be presented.
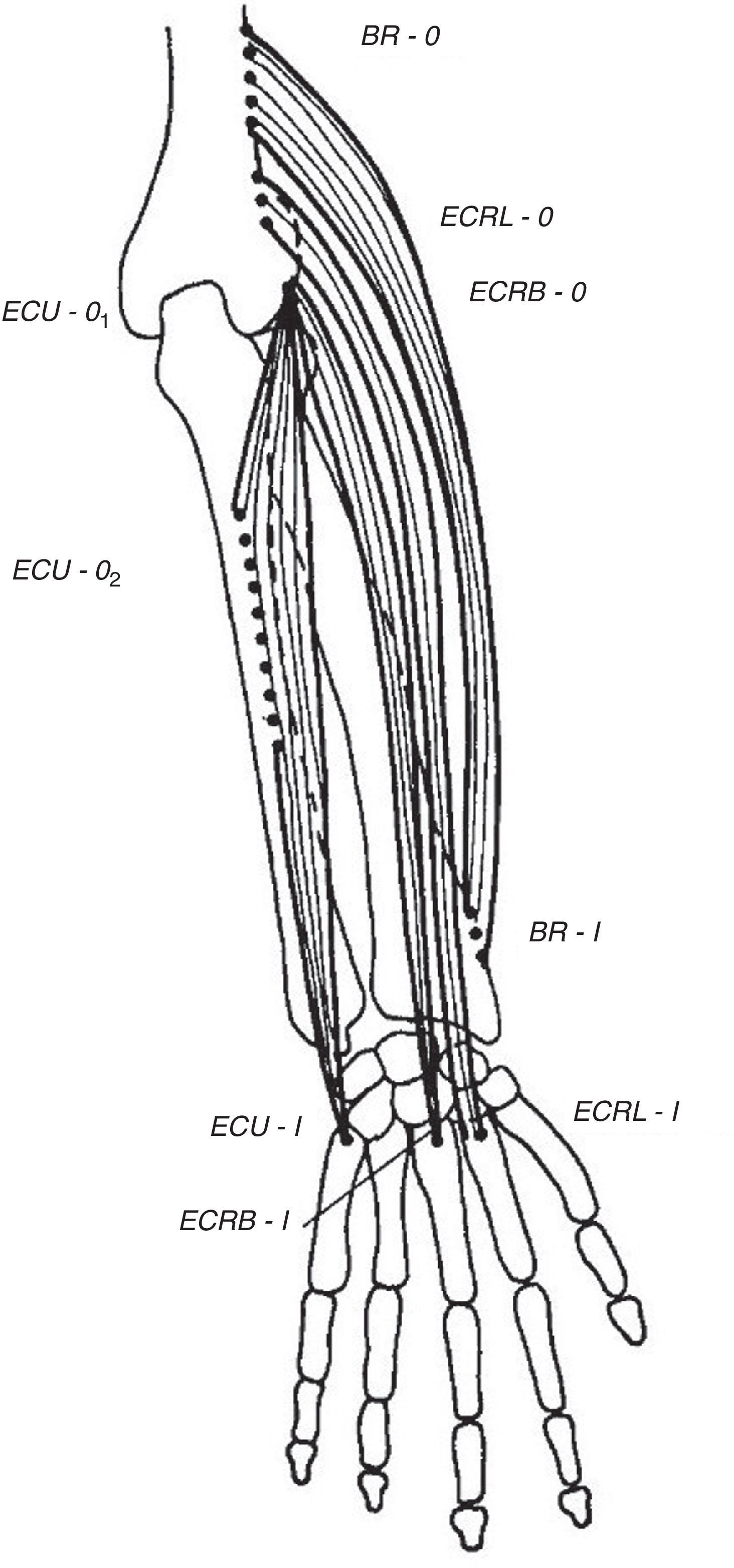
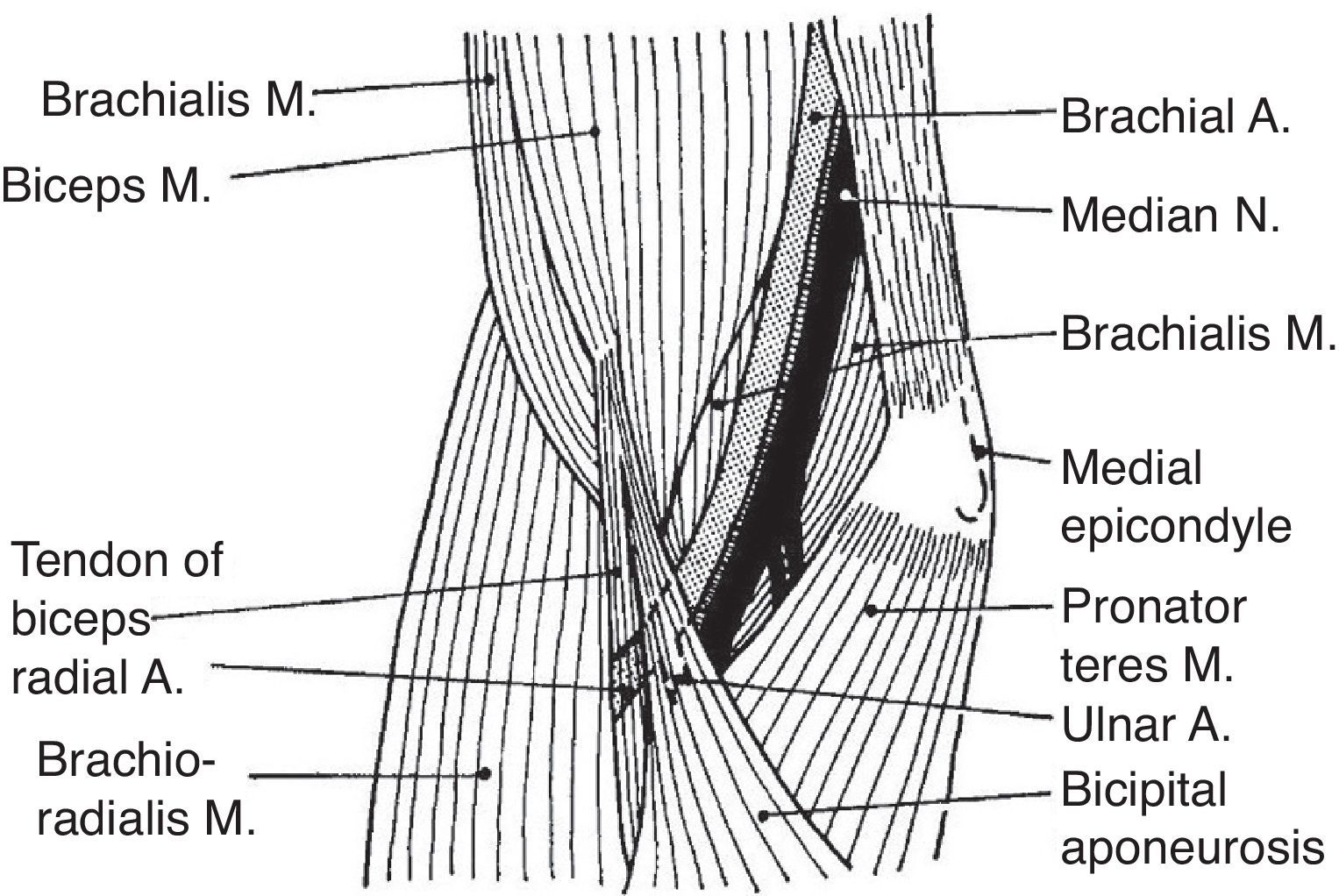
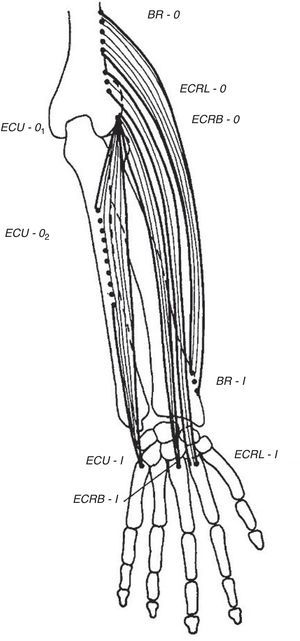
Wrist extensorsOf the muscles that participate in wrist extension, extensor carpi radialis longus (ECRL) has an origin in the lateral lower humerus, and extensor carpi radialis brevis (ECRB) and extensor carpi ulnaris (ECU) originate in the lateral epicondyle. The insertion of these muscles is in the dorsal base of the metacarpals 2, 3 and 5, respectively. Henry4 named the first two muscles, plus brachioradialis (BR) that has its origin in the lateral lower humerus and inserts in the radial styloid, the mobile wad (the mobile bundle) (Fig. 3). How to identify each of them? With the elbow flexed 90° and the thumb pointing up any resisted elbow flexion makes BR stand out. Follow its edge proximally the lateral lower humerus is reached. If in the same position the forearm is pronated and the wrist dorsiflexed the belly of ECRL bulges between ECR and the lateral epicondyle. ECRB however, appears to sink in a tendinous structure that turns fleshy farther down in the forearm. The origin of ECRB is the site of pathology in tennis elbow.5,6 This tendon suffers a degenerative process characterized by immature fibroblasts, the appearance of nonfunctional vascular buds and the presence of disorganized collagen that led Nirschl to coin the descriptive term “angiofibroblastic tendinosis”.7 Interdigitation between the origins of ECRB and that portion of ED that extends the 3rd digit explains Maudsley's test,8,9 which is pain upon resisted extension of the 3rd digit. There are several additional causes of lateral elbow pain (Table 1). An important differential diagnosis in refractory tennis elbow is a compressive neuropathy of the posterior interosseous nerve which is a deep terminal branch of the radial nerve. This condition typically features lateral elbow pain, pain in the first web space of the hand and weakness of the wrist and digital extensors.10 The nerve can be compressed anterior to the elbow joint or at the proximal edge of the superficial head of supinator (S) known as Frohse's arcade (FA)11,12 which can be mapped in the extended arm using the anterior elbow crease as a reference point. Published data indicate that this crease is about 2.2cm proximal to the radiohumeral joint13 and this joint, about 3.4cm proximal to FA.14 Thus, in the extended arm FA is 5.6cm distal to the elbow crease, or 5cm distal to the lateral epicondyle15 as the medial 2/3 meet the lateral 1/3 of the forearm.
The extensor muscles of the wrist. BR=brachioradialis, ECRL=extensor carpi radialis longus, ECRB=extensor carpi radialis brevis. ECU=extensor carpi ulnaris. O=origin, I=insertion.
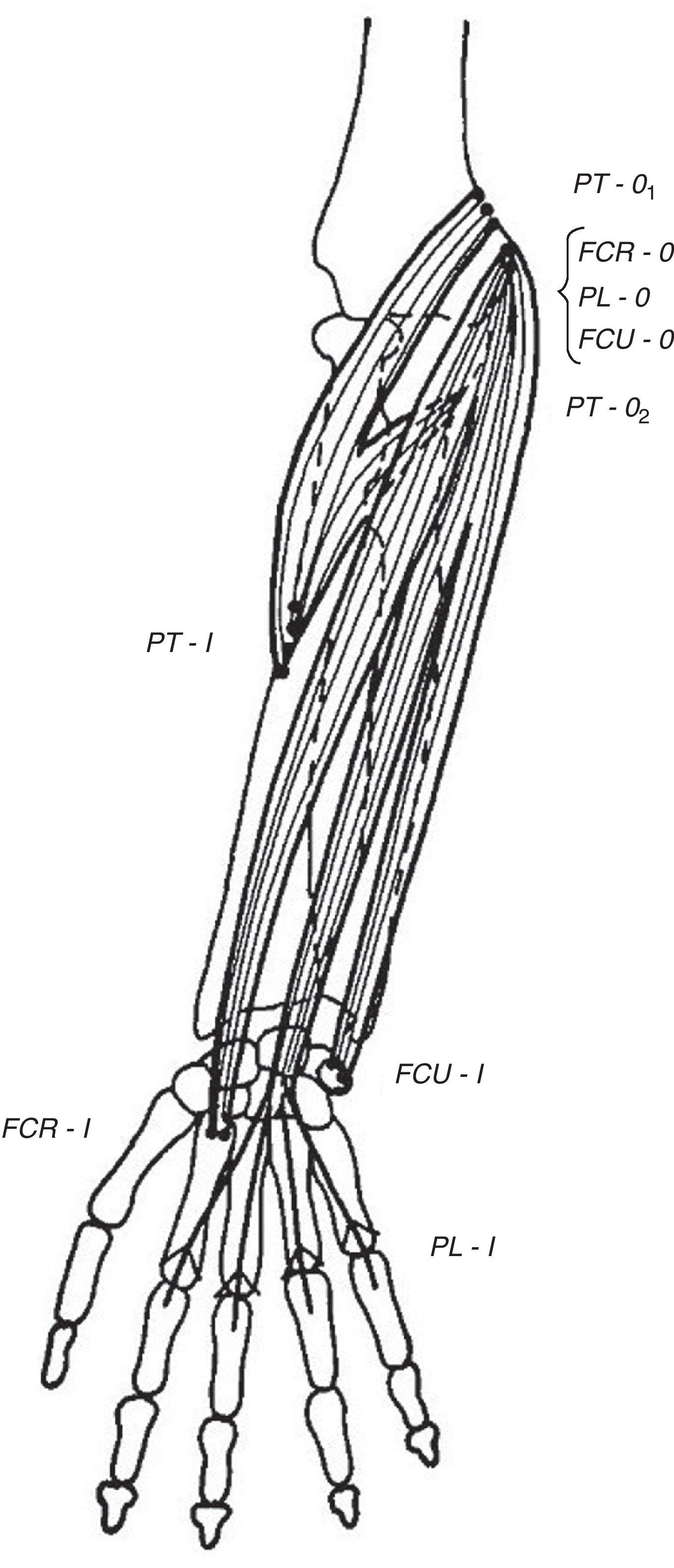
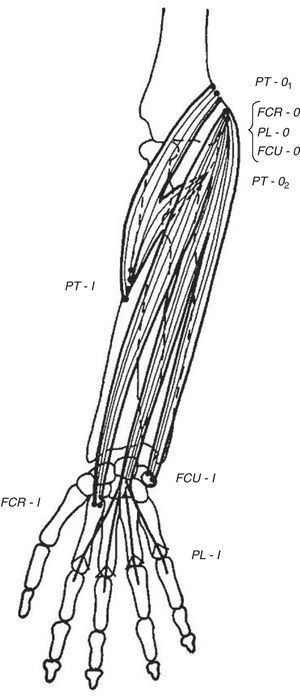
Wrist flexors, including flexor carpi radialis (FCR), palmaris longus (PL) – which is absent in approximately 10% of people – and flexor carpi ulnaris (FCU) run superficially in the ventral forearm (Fig. 4). Their origins are at, or close to, the medial humeral epicondyle. They insert at the base of the second metacarpal, the flexor retinaculum and palmar aponeurosis and the pisiform bone, respectively. These tendons can be made prominent in the distal forearm by resisted palmar flexion of the wrist. When PL is absent a hollow, underlied by flexor digitorum superficialis (FDS), appears ulnar to FCR. Pronator teres (PT) has two origins, the lower part of the medial supracondylar ridge and the medial epicondyle, and follows an oblique course in the upper ventral forearm (Fig. 4). The median nerve enters the forearm between the 2 heads of PT which is a well-known site for median nerve compression at the proximal forearm.16 Pronator teres inserts in the middle of the lateral surface of radius and its belly can be easily identified during pronation with the elbow at 90° flexion as a narrow triangular space that can be seen and/or felt having the biceps tendon (BT) at its base, radially the mobile wad and ulnarly PT. Notice that during pronation the distal end of radius swings around the head of ulna while the ulna abducts. This brings us to anconeus (A), a small muscle with a narrow origin at the lateral epicondyle and a broad insertion at the proximal ulna. As can be determined by simple observation and palpation, A appears to be responsible for the abduction of the ulna.17 Two additional actions of A can be readily shown by palpation. Feel A with a fingertip while the elbow is being actively extended. A contracts to assist triceps in elbow extension. Also, if the examiner's thumb is placed on P and the index on A, a synchronous contraction occurs during active pronation. A last point that is of great theoretical interest is the following: Percussion with the narrow end of a reflex hammer on the distal lateral head of triceps contracts fibers in anconeus that appear to be in continuity with the lateral triceps fibers.
The flexor muscles of the wrist. FCR=flexor carpi radialis, PL=palmaris longus, and FCU=flexor carpi ulnaris. PT=pronator teres is not a wrist flexor but partially shares their origins.
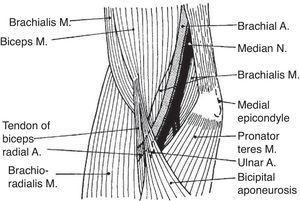
Biceps brachii (BB) and brachialis (B) are 2 muscles that cross the elbow anteriorly and are widely considered elbow flexors (Fig. 5). The biceps tendon, that joins the long and the short bicipital heads, is a narrow tendon that inserts in the posterior part of the radial tuberosity. Since the radius may rotate upon its longitudinal axis at the radicapitular joint, a medial pull will cause supination. However, on a supinated forearm BB acts as an elbow flexor. Another insertion of BB is fascial. The bicipital aponeurosis, also known as lacertus fibrosus, arises from BB just above the elbow crease and follows a medial and slightly anterior course where it merges with the fibrous forearm fascia. If one remembers its course the proximal bicipital aponeurosis can be readily palpated as a strong, thin, clearly demarcated edge. Deep to BB is B. Brachialis has its origin in the distal 2/3 of the front of the humerus and inserts in the tuberosity of ulna. Thus, B is a pure elbow flexor.
Brachialis, a muscle few think about, lies deep to biceps brachii. Brachialis inserts in the ulna and is a pure elbow flexor. Biceps brachii inserts in the medial tubercle of radius and as such is a forearm supinator and a flexor of the elbow joint. The bicipital aponeurosis, known by clinicians as lacertus fibrosus, is an insertion of biceps brachii in the antebrachial fascia.
Table 2 lists several causes of medial elbow pain that should be considered in the differential diagnosis of golfer's elbow. A very important one is an ulnar neuropathy. The ulnar nerve descends through the medial aspect of the arm anterior to the intermuscular septum. At 8-12cm from the epicondyle it changes to the back of the septum passing through the canal of Struthers. At 3.5cm from the epicondyle the ulnar nerve already lies in a sulcus between the medial epicondyle and the olecranon. At this site the nerve is exposed and can be subject to trauma and compression. Finally, the nerve enters the cubital tunnel as it runs under the aponeurosis of FCU. The roof of this tunnel is a thickened portion of the retinaculum known as the arcuate ligament (Osborne constriction band), a fibrous band between the humeral and ulnar heads of FCU. The base of the tunnel includes the joint capsule, the medial collateral ligament and the olecranon process. The arcuate ligament gets taught in elbow flexion and laxe in extension.18 The cubital tunnel is the most frequent entrapment site of the ulnar nerve19 and ulnar neuropathy at the elbow is only second to carpal tunnel syndrome as the most frequent compression neuropathy of the upper extremity. The clinical features of ulnar neuropathy at the elbow include elbow pain, numbness or tingling in the distribution of the nerve, nocturnal awakening and worsening of symptoms with elbow flexion.20 Motor symptoms of ulnar neuropathy at the elbow are less common and include loss of dexterity and weakness of intrinsic hand muscles.21 Ulnar-innervated intrinsic muscles include all dorsal and ventral interossei, the third and fourth lumbricals, all hypothenar eminence muscles, adductor pollicis and the deep head of flexor pollicis brevis.3 In long standing ulnar neuropathies the hand adopts a clawed position.
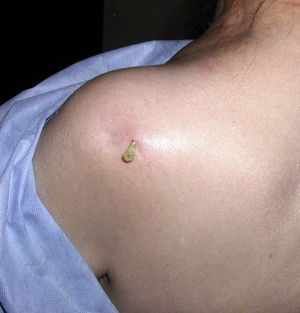
Patient 3. Olecranon bursitis “A 67 year-old man has an unsightly swelling at the tip of his right elbow”
This case brings us to the anatomy of the back side of the elbow. At this site the skin, from being smooth and elastic in young people, becomes loose and wrinkly and furrows in the shape of an inverted U proximal to the olecranon process appear in old age. Since we lean so much on the elbows it is surprising how infrequent skin tears are at this site. Protection against shear damage is afforded by two gliding mechanisms, the subcutaneous olecranon bursa and an abundance of areolar tissue. The olecranon bursa is a subcutaneous sac sparsely lined by synovial cells. No detectable fluid is present in the normal state. Different from deep bursae and diarthrodial joints, subcutaneous bursae cavitate during childhood and their size increases over time.22 The olecranon bursa lies between the skin and a firm base that includes the triceps (T) tendon, the back of the olecranon process and A. In bursal effusions internal pressures in the olecranon bursa increase with elbow flexion but not in extension.23 In contrast, in elbow joint effusions pressures are low in mid flexion and increase with further flexion and extension of the joint.24 Because the olecranon bursa lies so close to the skin, and because such a firm base supports it, common pathologies include traumatic bursitis from repetitive direct trauma and septic bursitis from infection caused by skin bacteria. Gout and rheumatoid arthritis are additional, well-known causes of olecranon bursitis.25 The olecranon process lies in the olecranon fossa in elbow extension. The synovial sac around this process, also with the shape of an inverted U, is a good site for elbow injection and aspiration. The ulnar side of the olecranon process must be avoided in these procedures because the ulnar nerve runs between this process and the medial epicondyle. As mentioned, there are two extensor muscles that attach to the olecranon process, triceps brachii (T) and A. T is a large, powerful muscle that comprises the entire posterior arm. It has two humeral heads, the lateral and the medial that flank the radial nerve as it descends down the spiral groove in the posterior humerus, and one long head that attaches to the scapular infraglenoid tubercle. These three heads converge into a common tendon that inserts into the posterior surface of the olecranon, and by way of a fibrous expansion to the superficial fascia of anconeus and the deep fascia of the forearm.26 Anconeus covers the posterior portion of the anular ligament, the radial head and the proximal ulna.
A distended olecranon bursa is often painless unless there is an associated acute inflammatory process. In such cases, the bursa may rupture and dissect the surrounding subcutaneous tissue in the arm and forearm27; a recurrent edematous swelling of the forearms has also been described presumably due to chronic leakage.28
Patient 4. Elbow injection, lateral approach “A 34 year-old woman with rheumatoid arthritis has progressive monoarticular activity and flexor contracture in her left elbow”.
Few experienced rheumatologists would argue against using intraarticular steroids in a scenario such as the one presented. Focusing on the clinical anatomy of the radio-humeral joint we use this case to review the lateral approach to an intraarticular elbow injection. The elbow is comprised of three different joints that share a single synovial cavity: the humeroradial, the humeroulnar and the proximal radioulnar joints. The joint that concern us in this case is the humeroradial between the humeral capitulum and the head of the radius. This joint, in conjunction with the distal radioulnar joint, provides for pronation and supination.29
We suggest, for blind injections of the elbow, a lateral approach: with the elbow flexed at 90° resting on a firm surface small pronation and supination movements allow to distinguish the unmoving lateral epicondyle and the rotating head of radius. This cleft is marked. An insulin needle is then vertically inserted through a front of anesthesia. Free flow of the anesthetic solution at a depth of 7-10mm indicates an intraarticular placement of the needle (Fig. 6).30
- •
Lateral epicondyle
- •
The mobile wad: braquioradialis, extensor carpi radialis longus and extensor carpi radials brevis
- •
Extensor carpi ulnaris
- •
Medial epicondyle
- •
Flexor carpi ulnaris
- •
Palmaris longus
- •
Fexor carpi radialis
- •
Pronator teres
- •
Biceps brachii
- •
Bicipital aponeurosis
- •
Brachialis
- •
Supinator
- •
Frohse's arcade
- •
Struthers canal
- •
Ulnar groove
- •
Cubital tunnel
- •
The elbow flexion maneuver for ulnar nerve entrapment at the elbow
- •
Ulnar nerve palpation in extension and flexion of the elbow
- •
Olecranon process
- •
Triceps tendon
- •
Triceps, the scapular and the two brachial heads
- •
The spiral groove for the radial nerve
- •
Anconeus
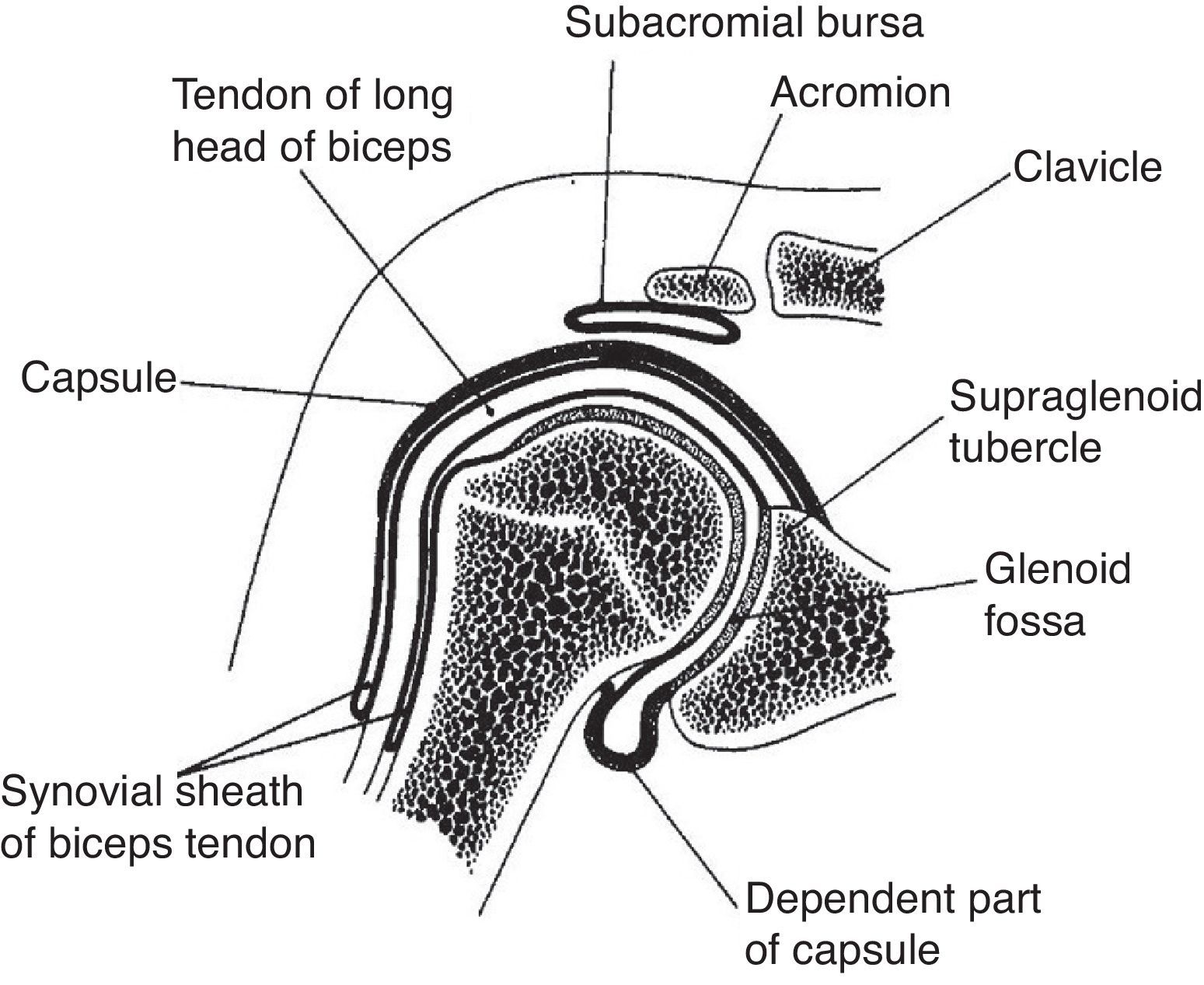
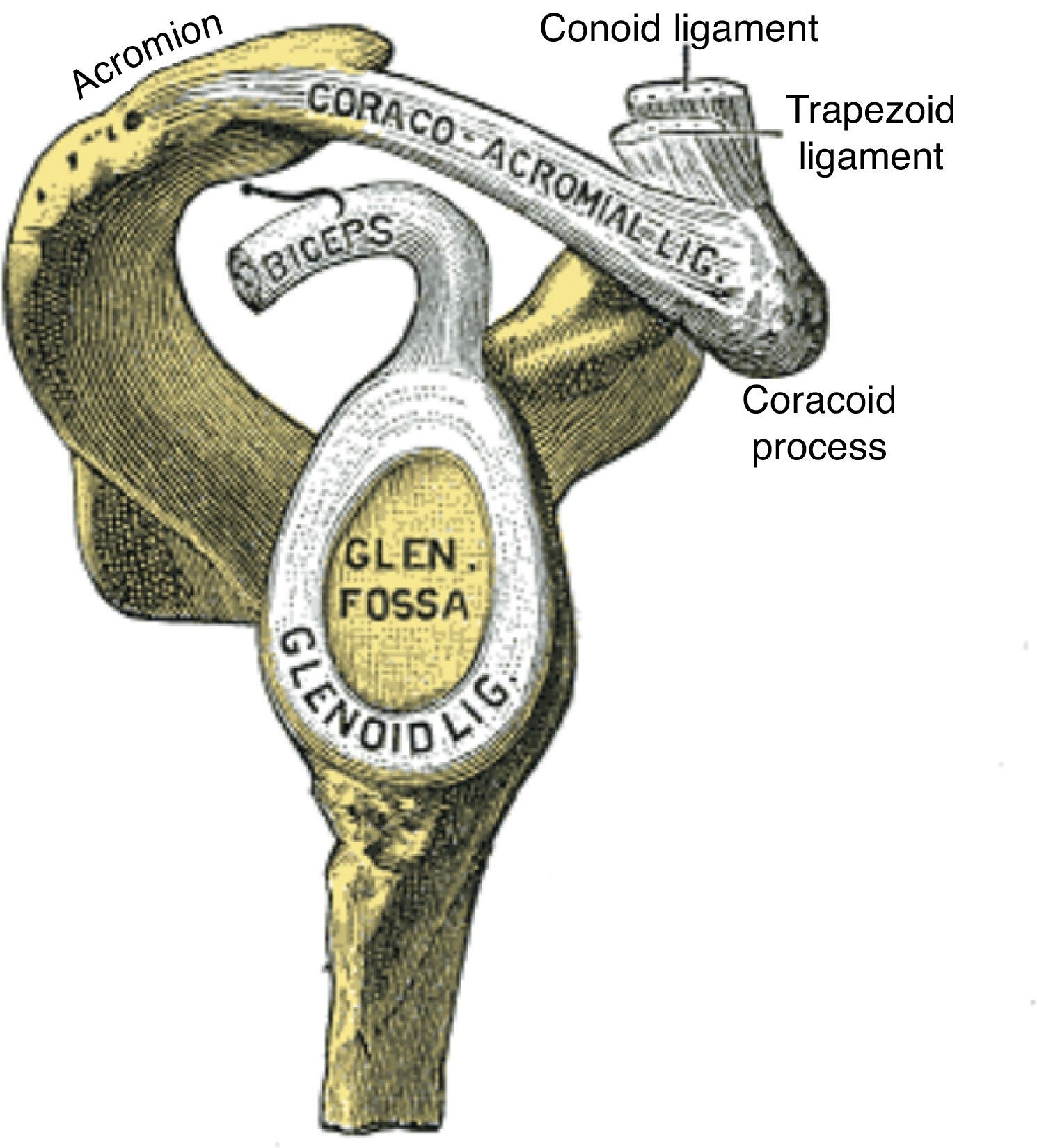
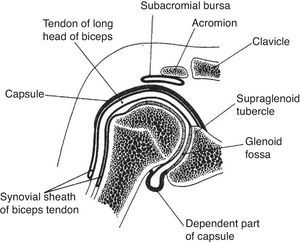
The GH joint is formed between the humeral head and the glenoid fossa. The average vertical dimension of the humeral head is 48mm with a radius of curvature of 25mm and an average transverse dimension of 45mm with a radius of curvature of 22mm. The oval concave glenoid surface has an average vertical dimension of 35mm and a transverse diameter of only 25mm (Fig. 7).31 Static stabilizers of the GH joint include its articular surfaces, the labrum glenoidale, the joint capsule, the coracohumeral ligament, and the glenohumeral ligaments. The shallow glenoid fossa is augmented by a fibrous and partially fibrocartilaginous rim, the labrum glenoidale32,33 which is only loosely attached to bone in the upper half but has a firm attachment in the lower half (Fig. 8). The inner surface of the labrum is covered by synovium that is continuous with the adjacent capsular synovium. The long head of biceps inserts in the supraglenoid tubercle and in the labrum while the long head of triceps attaches to the infraglenoid tubercle and adjacent border of the scapula.34 Anteroposterior fissures frequently develop in the upper portion of the labrum and have been named SLAP (superior labral antero posterior) lesions.35 These may vary in extent and may or not compromise the labral insertion of the long head of biceps. Patients with SLAP lesions complain of shoulder pain that increases with overhead activities plus a painful “catching” or “popping” sensation in the shoulder. Another presentation of SLAP lesions is the “dead arm” syndrome in which sharp pain and discomfort at the beginning of acceleration results in a suboptimal performance of the throwing athlete.36 Occasionally a synovial cyst develops from leakage of synovial fluid. These cysts tend to grow into the spinoglenoid notch and cause a suprascapular nerve compression neuropathy. Three glenohumeral ligaments, the superior, the middle and the inferior reinforce the anterior part of the capsule with the addition of the strong coracohumeral ligament.37–40 The thickest portion of the capsule is a ring 1-2mm thick solidly welded to the rotator cuff near its actual insertion.38 This ring has been named the “rotator cable”.41 Dynamic stabilizers include the deltoid muscle, the rotator cuff muscles and the long head of biceps. Interestingly, the triceps tendon significantly reinforces the posteroinferior GH capsule and probably makes a contribution to static stability.34,38 Although static stabilizers are important, as shown by the serious shoulder pathology that often characterizes patients with joint hyperlaxity, GH joint stability is for the most part provided by the rotator cuff muscles.31,32
A frontal section of the glenohumeral joint. The humeral head articulates with the glenoid. The long head of biceps has its origin in the supraglenoid tubercle. In the lower portion of the joint the dependent part of capsule or axillary recess is seen. The subaromial bursa separates the acromion from the rotator cuff which is not shown. The long head of biceps tendon exits the glenohumeral joint surrounded by a synovial sheath.
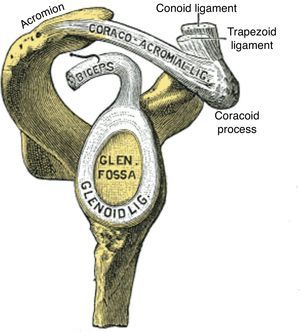
Lateral view of the scapular components of the glenohumeral joint. The labrum glenoidale (glenoid ligament) in continuity with the tendon of the long head of biceps is shown. The acromion, the coracoacromial ligament and the coracoid process form the coracacromial arch that protects superiorly the glenohumeral joint.
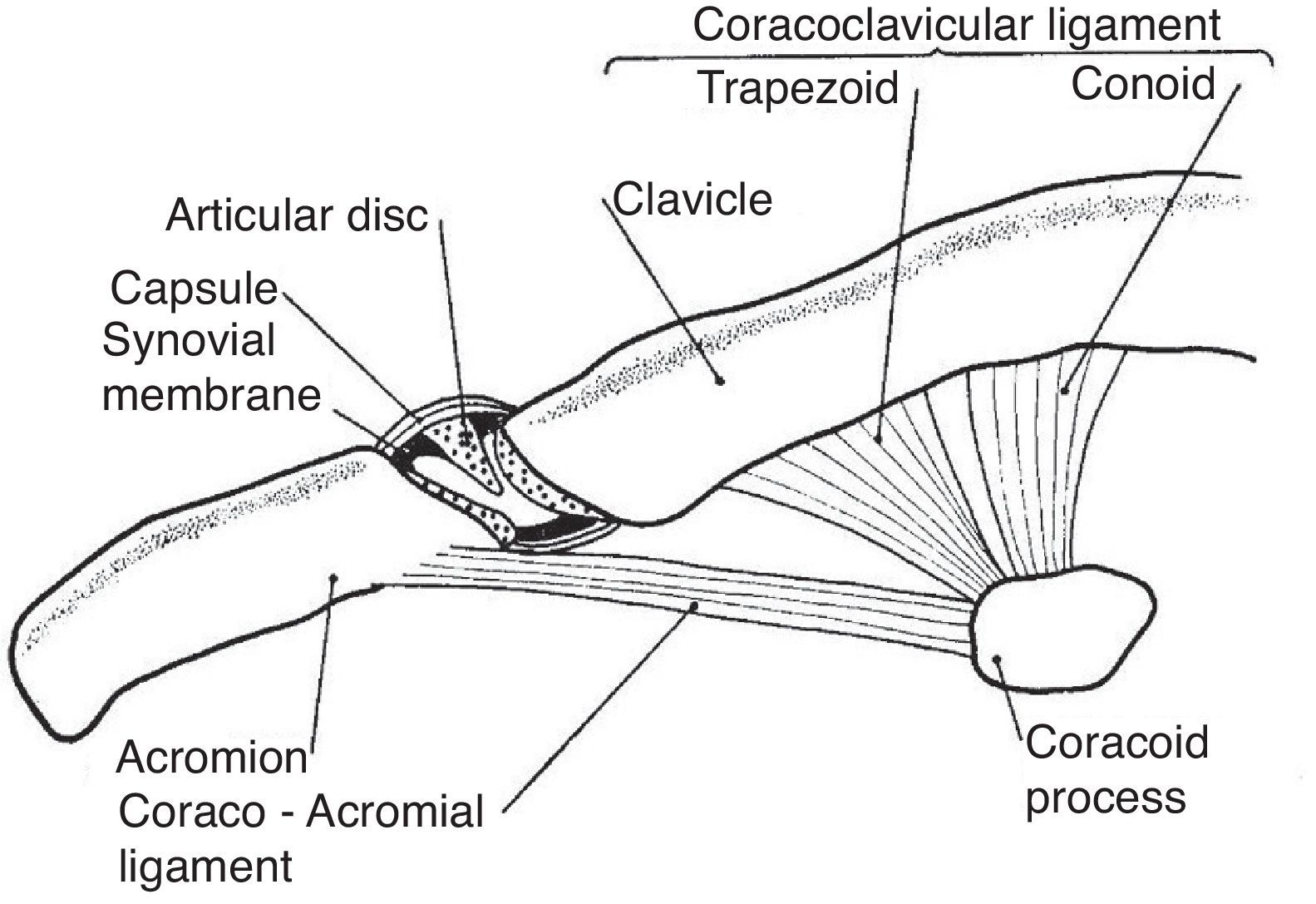
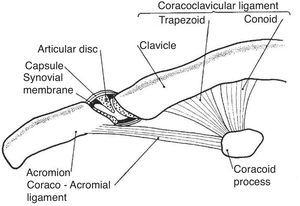
The shoulder girdle, a moving platform for the upper extremity, is formed by two bones: the clavicle and the scapula. These bones are kept together by the strong coracoclavicular ligaments and the diarthrodial, planar AC joint and its ligaments (Fig. 9). The AC joint has a fibrous capsule that is reinforced by the superior acromioclavicular ligament. Two movements of this joint, as seen from the scapular side, can be distinguished as the arm is abducted 90°, elevation of about 15° along the AP axis and internal rotation of about 6° around a vertical axis.42 Past 90° abduction an increasing upward rotation and posterior tilting of the scapula across the AC joint places increasing deformation and results in pain in the abnormal joint. Hence the terminal pain in the arc of elevation maneuver.43 The AC joint motion does not occur in isolation but is coupled with StCl motion which includes elevation, depression, protraction, retraction, and rotation of the clavicle around its axis. During arm elevation, clavicular motions include elevation, retraction, and posterior axial rotation44 caused by pull of the acromioclavicular and coracoclavicular ligaments as the scapula is actively moved around the chest. The AC joint is frequently affected in osteoarthritis and calcium diphosphate deposition (CPPD) disease, has an incomplete meniscus and a strong capsule and is felt as a “step down” as the clavicle is palpated distally. On the other hand the border may be sharply raised in osteoarthritis or be convex, smooth and very tender in inflammatory effusions. Past this step is the acromion which appears as a flattened surface whose lateral border ends posteriorly in the acromial angle. This prominent landmark is continuous with the spine of the scapula. A line connecting both scapular spines crosses the 3rd thoracic spinous process. Below the lateral border of the acromion the greater tubercle of the humerus and attached tendons can be easily palpated. The cleft between the acromion and the greater tubercle represents an excellent lateral access for injection of the subacromial bursa which underlies the anterior halve of the acromion. Approximately 2cm below the clavicle, in de deltopectoral groove, the tip of the coracoid process is felt. The coracoid and the head of the humerus may be distinguished from each other by a simple maneuver. With the arm hanging on the side the subject is asked to externally and internally move the arm in a repetitive fashion. Palpation will distinguish the rotating humeral head from the coracoid process that remains steady. This is the rotator interval area where the coracohumeral ligament is palpable as a transverse bar when the arm is in external rotation.
The acromioclavicular joint. This joint has an incomplete disc or meniscus. The ligaments that connect the coracoid to the clavicle, conoid and trapezoid are also shown.
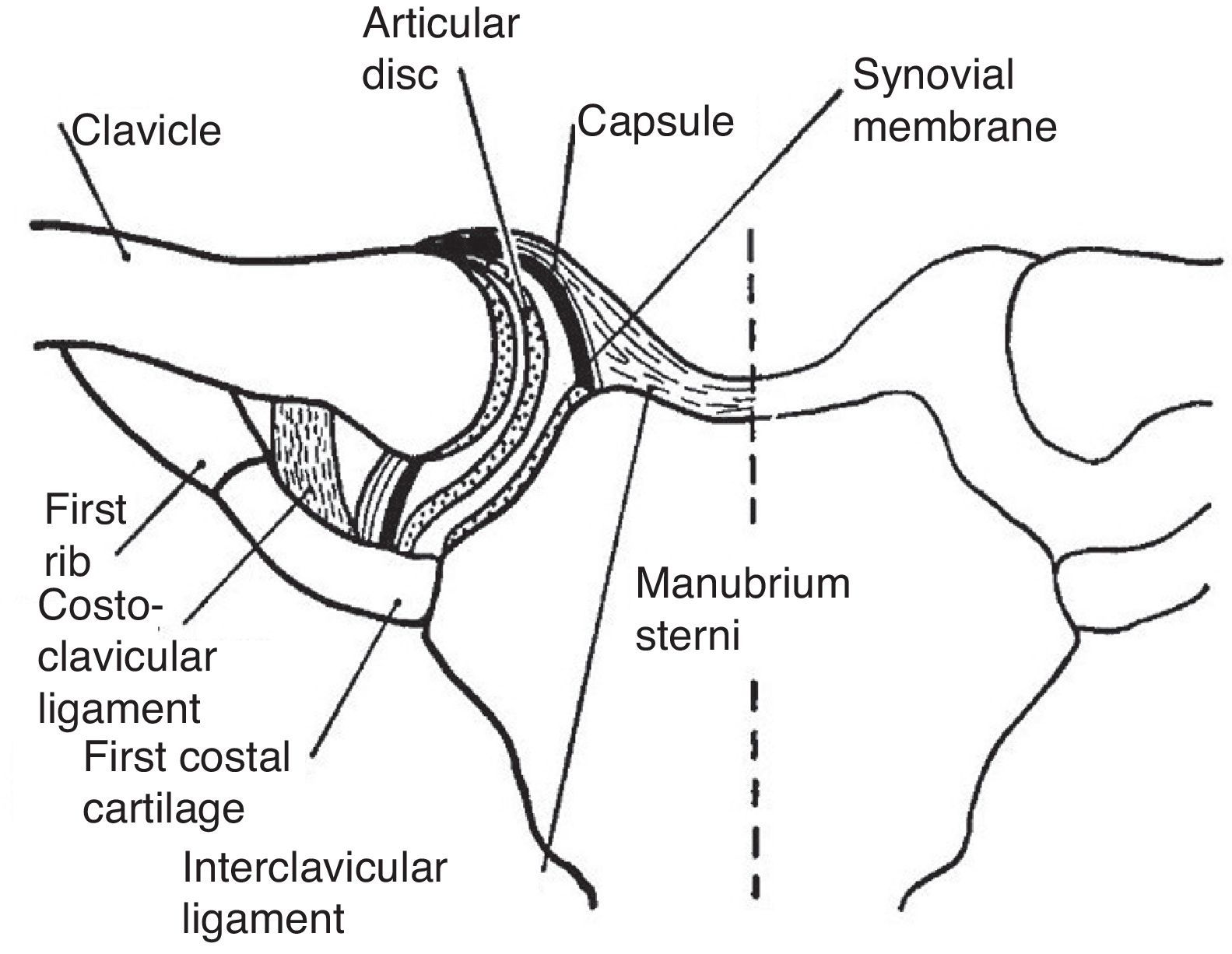
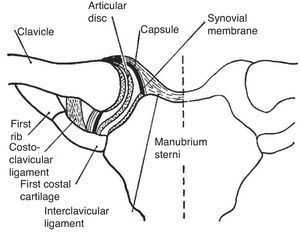
The StCl joint has a thick capsule and a complete meniscus lined with hyaline cartilage (Fig. 10).45 The scapulothoracic motion can be easily documented by placing the fingers of the exploring hand on the scapular spine and the thumb in its inferior angle. The person is then asked to slowly abduct the arm. Anterolateral displacement of the inferior angle, along with a counter-clockwise rotation of the scapular spine will be perceived throughout abduction. A similar scapular displacement occurs with forward flexion. This counter-clockwise motion, known as the scapulothoracic mechanism, elevates the glenoid fossa and allows the arm to operate from a variable, finely controlled platform that can not only be raised, but also moved along parallels at any degree of abduction. It is quite important to prevent, while clinically assessing GH joint motion, the scapulothoracic motion. This is done by placing one hand flat on the shoulder to fix the clavicle and the scapula while the other hand passively elevates the arm. Another important concept in the evaluation of shoulder motion is the scapular plane. The scapula, a plane bone, is paced at a 30° angle with the coronal plane when the arm is hanging on the side. Since the humerus must be in line with the scapula, abduction and adduction occur 30° anterior and 30° posterior to the coronal plane, respectively. On the other hand, flexion and extension occur 30° past and 30° short of the sagittal plane, respectively.46
The sternoclavicular joint. This joint is partitioned by a complete disc or meniscus. The ligaments that bind and connect this joint to the 1st rib are shown.
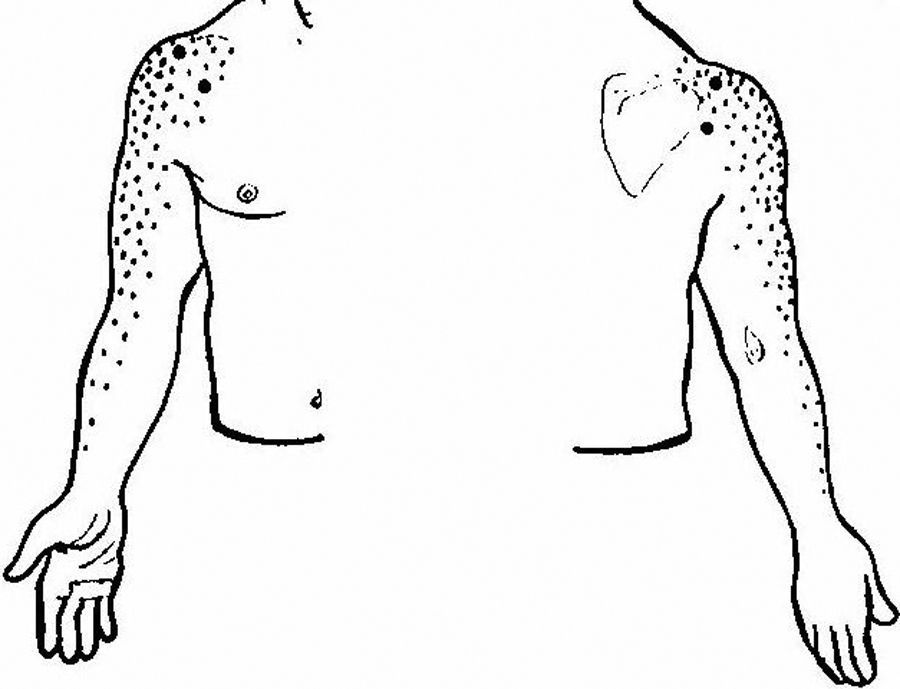
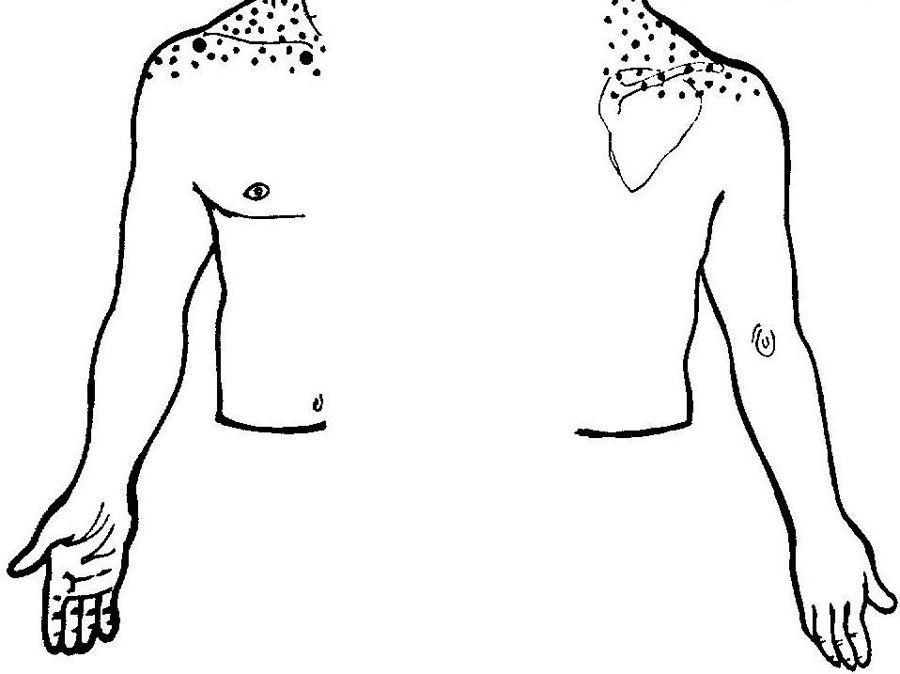
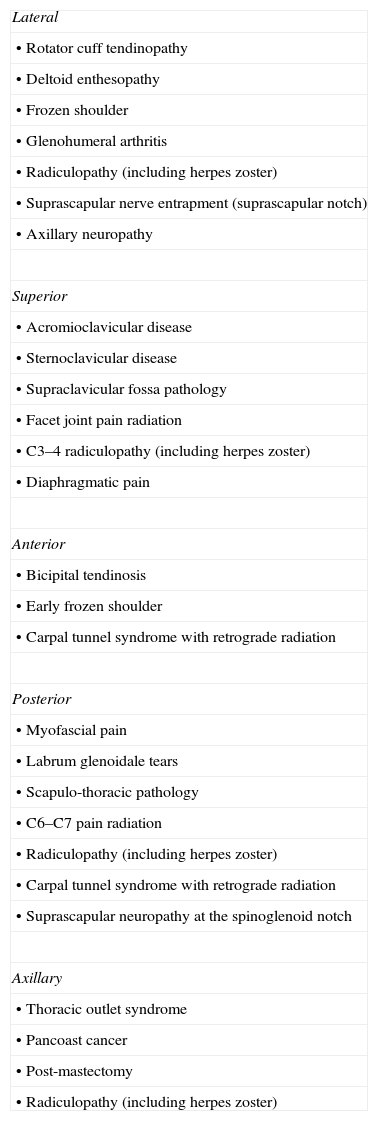
As an initial step patients with shoulder pain should be asked to point to the area of maximal pain. This simple question narrows down significantly the differential diagnosis (Table 3). Most patients with shoulder pain have either lateral pain or superior pain (Figs. 11 and 12). In deep structures such as the rotator cuff and the glenohumeral (GH) joint pain is experienced laterally into the deltoid muscle area and may even spread farther down following the radial side of the extremity. Superficial structures such as the acromioclavicular (AC) and the sternoclavicular (StCl) joints hurt locally and may have a small adjacent referral area that for the StCl joint is in the supraclavicular region. On the whole pain that is referred from deep structures appears to follow structures of similar segmental derivation.47 Thus, since the rotator cuff and the GH joint derive from the 5th and 6th cervical segments, referred pain involves the deltoid muscle and overlying skin. For the AC and StCl joints, which derive from the C4 segment, pain is experienced locally in the former and locally, in the supraclavicular region and in the lateral neck in the latter. Needless to say, supraclavicular pain may originate in the neck. The very common upper trapezius and scalene pain, which is usually caused by muscle contracture, is triggered by contralateral bending the neck. In contrast, cervical spine pain is triggered by homolateral flexion of the neck. Finally, because the tendinous center of diaphragm is innervated by the C3-derived phrenic nerve, supraclavicular pain may have a diaphragmatic origin as well. A golden rule in shoulder examination is to compare active and passive motion. In tendinosis, as in our Patient 5, while passive motion is complete and virtually painless, resisted motion causes pain and inhibition weakness. In contrast, in disease processes of the GH capsule or other passive stabilizers both active and passive motions are restricted and the endpoint may be painful as in our Patient 6. Is the insertion of the rotator cuff palpable? With the arm in extension the greater tubercle moves forward and exposes the supraspinatus. If the arm in the same position is now internally rotated the infraspinatus insertion is exposed. In addition to these classic maneuvers we would like to propose the following: with the arm hanging on the side first identify the coracoid process and then rotate the arm externally. This makes the coracohumeral ligament37 taut and palpable in most people. Perhaps this maneuver would be useful in the diagnosis of an early frozen shoulder in which swelling and tenderness of the ligament would be expected. Indeed, we recently saw a patient with an early unilateral frozen shoulder in whom the coracohumeral ligament was swollen and tender in the limited side. Another golden rule is that if a patient is asked to raise the arms in the scapular plane, in rotator cuff tendinosis there is pain in the middle third of elevation while in acromioclavicular disease there is mounting pain in the upper third of elevation.43 Thus, pain location, pain on active motion or at the end of passive motion and a careful evaluation of the arc of elevation maneuver provide a firm basis from which to build a clinical diagnosis in patients with shoulder pain.
Shoulder pain according to localization.
| Lateral |
| • Rotator cuff tendinopathy |
| • Deltoid enthesopathy |
| • Frozen shoulder |
| • Glenohumeral arthritis |
| • Radiculopathy (including herpes zoster) |
| • Suprascapular nerve entrapment (suprascapular notch) |
| • Axillary neuropathy |
| Superior |
| • Acromioclavicular disease |
| • Sternoclavicular disease |
| • Supraclavicular fossa pathology |
| • Facet joint pain radiation |
| • C3–4 radiculopathy (including herpes zoster) |
| • Diaphragmatic pain |
| Anterior |
| • Bicipital tendinosis |
| • Early frozen shoulder |
| • Carpal tunnel syndrome with retrograde radiation |
| Posterior |
| • Myofascial pain |
| • Labrum glenoidale tears |
| • Scapulo-thoracic pathology |
| • C6–C7 pain radiation |
| • Radiculopathy (including herpes zoster) |
| • Carpal tunnel syndrome with retrograde radiation |
| • Suprascapular neuropathy at the spinoglenoid notch |
| Axillary |
| • Thoracic outlet syndrome |
| • Pancoast cancer |
| • Post-mastectomy |
| • Radiculopathy (including herpes zoster) |
Patient 5. Rotator cuff tendinopathy “A 40 year-old house painter has recurrent lateral right shoulder pain. Passive range of motion is painless and has normal range but severe pain and weakness occur in resisted abduction and external rotation”
Patient 6. Frozen shoulder “A 60 year old woman with poorly controlled diabetes is seen with painful restriction of the right shoulder. Two months prior an anterior right shoulder pain that woke her up at night had its onset. Within a month pain extended to the deltoid region and progressive limitation of the joint ensued. She had on examination a 20° abduction and 0 external rotation, severe pain on the endpoint, a normal ESR, a glucose level of 200mg/dl and a normal looking shoulder in an AP X ray film of the right shoulder”
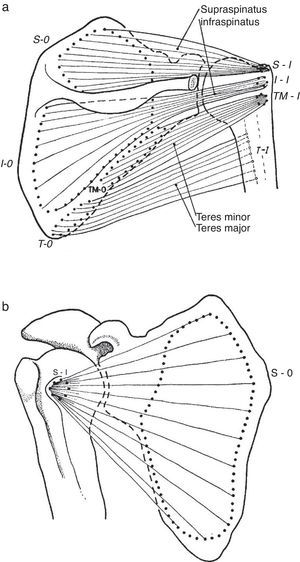
Patient 5 exemplifies a condition known as rotator cuff tendinosis which clinically features recurrent pain and weakness caused by an overhead activity, a normal passive range of motion and, in his case, pain upon the resisted action of supraspinatus (abduction) and infraspinatus (external rotation). The older designation used for this condition, tendinitis of the rotator cuff, is inappropriate because histopathologically the lesion lacks the usual changes of inflammation. Instead, vascular proliferation, fibrosis, mucin deposit and often fragmentation are present, with tendon rupture at the end of the pathogenetic chain.48 Rotator cuff tendinosis is the most common disorder of the shoulder49 and relates to anatomical characteristics of the short rotator muscles and the heavy work placed upon them. Indeed, salient risk factors for rotator cuff tendinopathy include age and an abduction position of the arm at work.50 The rotator cuff muscles are teres minor, infraspinatus, supraspinatus and subscapularis (Fig. 13a and b). These muscles originate in the scapula and prior to their insertion in the humeral tubercles their tendons splay out in a continuous, interdigitated cap known as the rotator cuff.38,51 Taken in isolation the first two muscles cause external rotation, the third, abduction and the fourth, internal rotation. The rotator cuff covers most of the humeral head. In turn, the whole shoulder joint is covered by the three portions of the deltoid muscle. The cuff muscles, in addition to providing rotational power to the humerus, center and retain the humeral head against the glenoid fossa. Being their insertions so close to the axis of motion, i.e. as compared with the humeral insertion of deltoid, they are subject to wide angular changes and cumulative damage during motion can be predicted. In the overhead position, cuff impingement may occur between the humeral head and the overlying coracoacromial arch.31 From down up, the subacromial space is occupied by the GH capsule, the supraspinatus tendon, the subacromial bursa, and the coracoacromial arch which includes the acromion, the coracoacromial ligament and the coracoid. In the 1940s and 1950s De Palma showed that the subacromial bursa does not communicate with the glenohumeral joint except in old age when partial tendon ruptures in over 50% and complete tears in about 15% occur. Additionally, he noticed that degeneration of the glenoid labrum and the long head of biceps was commonplace past the 6th decade of life.39 These findings, that many questioned at the time, have all been confirmed by US52 and MRI53 studies in the shoulders of asymptomatic subjects. A causal relationship between subacromial impingement54 and rotator cuff tendinosis has long been a matter of debate and indeed “shoulder impingement” as a broad disease category is loosing grounds to the non-committal designation “rotator cuff tendinopathy”. This is a plausible trend, as a name that solidifies a pathogenetic theory is being replaced by the descriptive terms tendinosis, partial tear or complete tear of the rotator cuff, all of which can be shown by US or magnetic resonance imaging. However, in terms of causality, avascularity, tensile overload, age-related degeneration, and impingement may all play a role in rotator cuff tendinosis.55
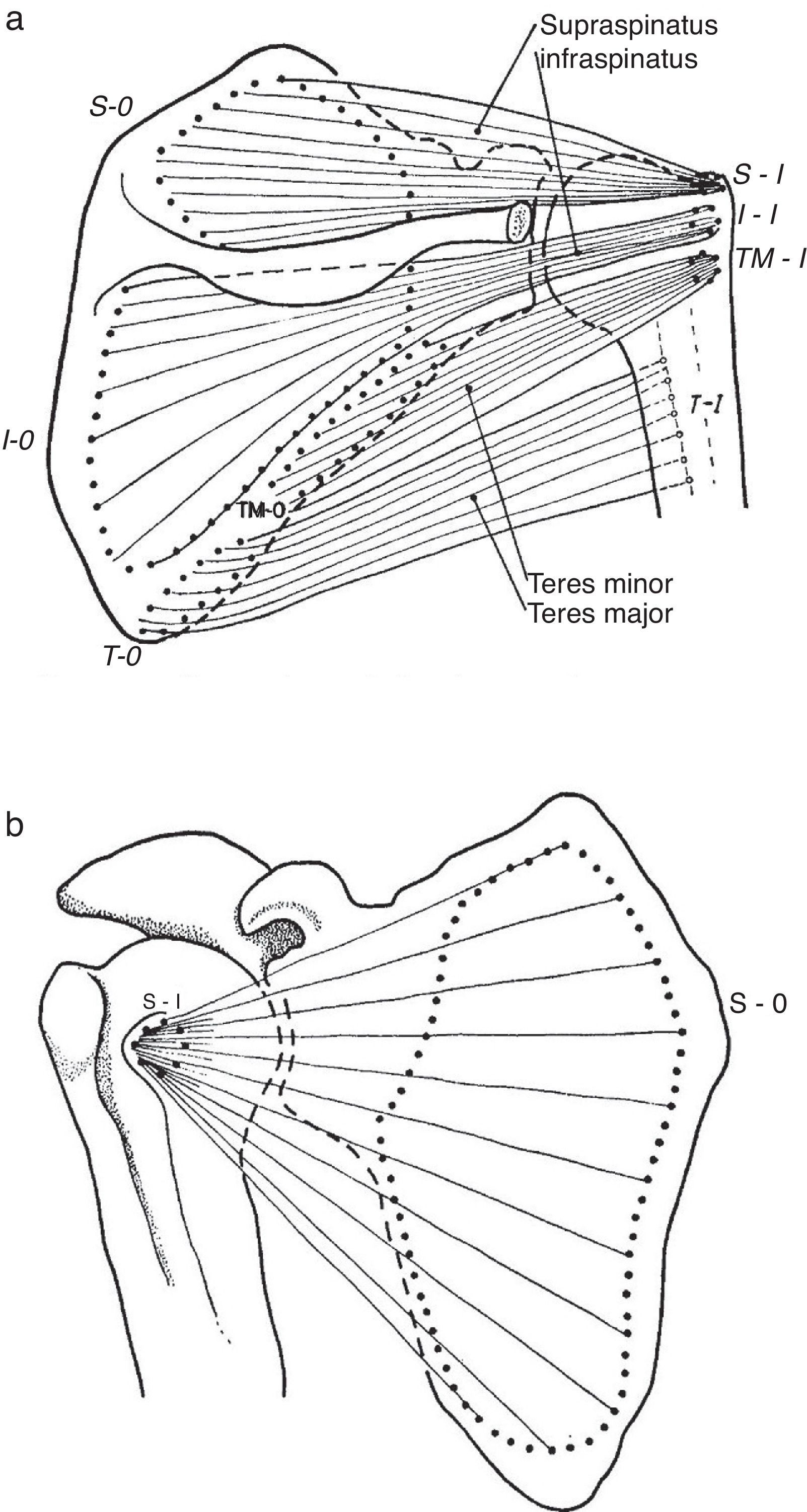
(a) Three of the rotator cuff muscles, supraspinatus, infraspinatus and teres minor are shown along with their origins (O) and their insertions (I). Teres major, which is not part of the cuff, is also shown. An interesting feature of teres minor and major is that they have opposite actions on the humerus. While the former is an external rotator, the latter is an internal rotator of this bone. (b) Subscapularis muscle has a fleshy origin in the scapula and a discrete insertion in the lesser tubercle of the humerus.
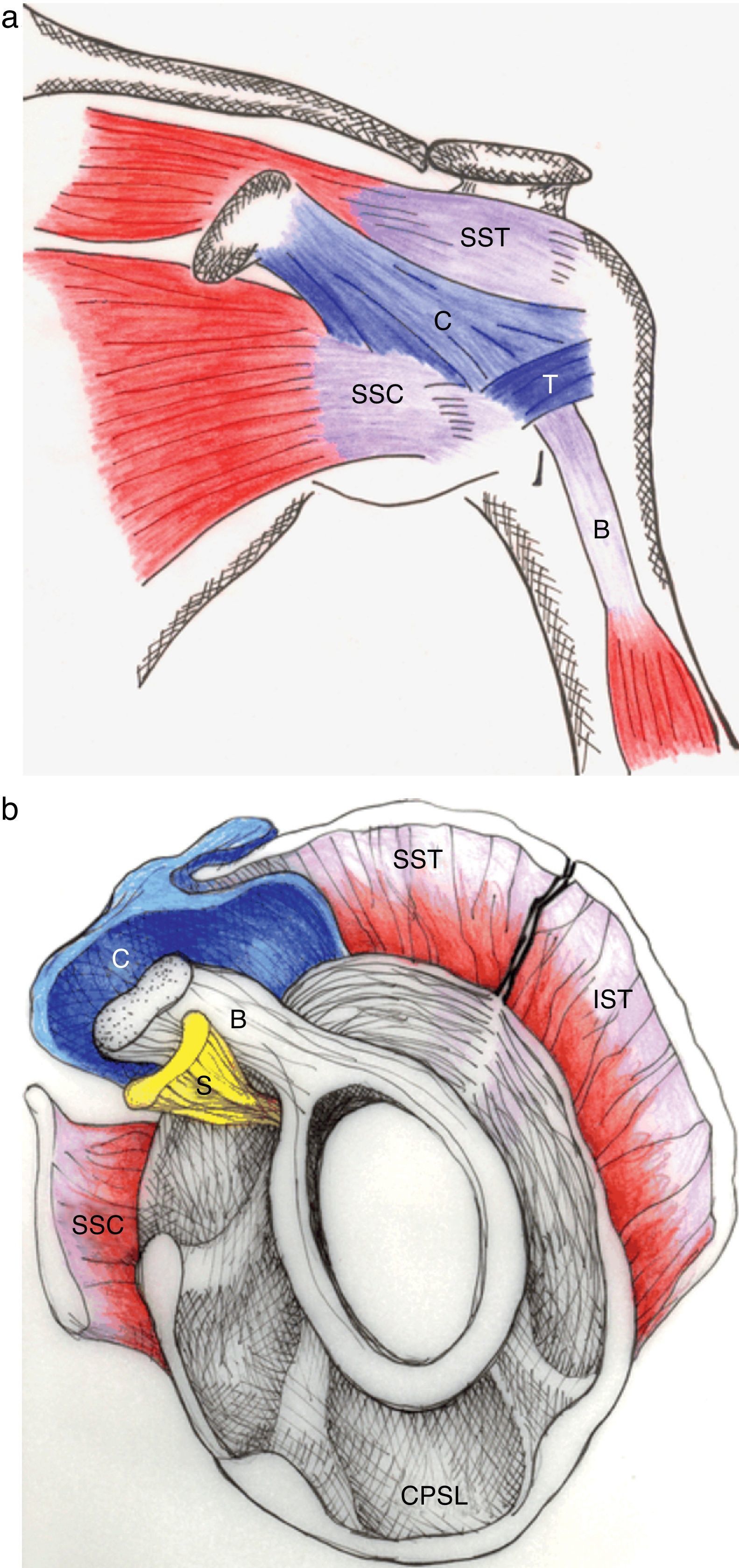
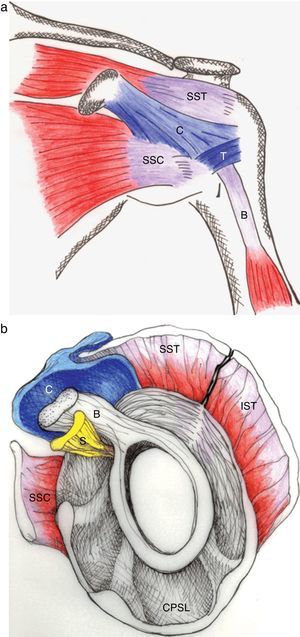
The pathology of the frozen shoulder has been investigated since the advent of arthroscopy which has allowed, on the one hand, to visualize tissue retractions, and on the other, to obtain biopsies of a previously poorly understood condition.56 In Robinson's review, the key clinical finding in frozen shoulder is a selective restriction of passive external rotation of the shoulder in adduction. This finding suggests tightness of the coracohumeral ligament in the rotator interval (Fig. 14a and b) which also includes the superior glenohumeral ligament and the interval capsule. Tightness of the inferior capsule and inferior glenohumeral ligament limits external rotation in abduction. Finally, in severe cases of frozen shoulder the posterosuperior capsule may be thickened and this limit passive internal rotation. Pathologically, at onset there is mild inflammation that gives way to a fibrotic process that reaches a maximum within weeks or months and then undergoes spontaneous, albeit incomplete, resolution. In most patients no underlying condition is found. Many patients have diabetes, however, and the theory has been that poorly controlled hyperglycemia leads to excessive glycosylation of Type I collagen making these molecules resilient to homekeeping degradation. However, levels of HbA1c were no different between diabetic patients with or without a frozen shoulder, weakening this assumption.57 Diabetic frozen shoulder patients tend to have a more severe and protracted disease course. From the viewpoint of the shoulder restriction the most significant finding is thickening of the coracohumeral ligament and interval capsule58 and surrounding hyperemia.59 A second finding is a disappearance of the axillary fold of the joint capsule, again with surrounding hyperemia.59 This brings us to a discussion of the rotator interval60 and the dependent portion of the GH joint capsule. If the rotator cuff is cut along its tubercular insertion the 4 converging muscles plus the coracohumeral ligament are evenly distributed at the edge of the tendinous cap.38 The rotator interval is an area between the anterior border of supraspinatus and the superior border of subscapularis which is not covered by rotator cuff tendons. Instead, this interval is sealed by a strong ligament, the above mentioned coracohumeral ligament which arises in the lateral aspect of the coracoid process, merges with the interval capsule and inserts in the lesser and greater tubercles of the humerus.37,38,61,62 The analogy of the coracohumeral ligament with the iliofemoral ligament of the hip is striking.37 The long head of the biceps tendon lies deep to this ligament on a groove between the greater and the lesser humeral tubercles. In addition to stabilizing the GH joint, the individual muscles of the rotator cuff provide the strength needed for arm movement. Pain upon their resisted actions is useful to determine the integrity of their tendons. The isolated assessment of tendon components is, however, limited by interdigitation of the insertional fibers of adjacent muscles which tends to blur the physical findings.38
(a) The coracohumeral ligament (C) inserts in the coracoid and extends laterally to the humerus covering the long head of the biceps tendon. Its insertion is in the lesser and greater tubercles of the humerus and in the fascia of the subscapularis (SSC) and supraspinatus (SST) muscles. T is the transverse humeral ligament that keeps the biceps tendon in the intertubercular groove. (b) As seen in this sagittal section, the coracohumeral ligament fills the space between the upper edge of subcoracoid (SSC) and the anterior edge of supraspinatus (SST). This gap, which includes the tendon of the long head of biceps (B) and the superior glenohumeral ligament (S) is widely known as the “rotator interval”.
In addition to the four muscles of the rotator cuff several muscles arise from the shoulder girdle and pass to the arm and forearm. These include teres major, deltoid, pectoralis mayor, biceps brachii with its two heads, coracobraquialis and the long head of the triceps. The powerful deltoid covers the glenohumeral joint on all sides except inferomedially and forms the smooth contour of the shoulder. This muscle has three different origins: the clavicle anteriorly, the acromion laterally and the scapular spine posteriorly; its fibers merge in a short V-shaped tendon that attaches to the lateral side of humerus. The different parts of deltoid can act autonomously as well as together. The anterior fibers draw the arm forward and assist internal rotation. Posterior fibers act with latissimus dorsi and teres major in drawing the arm backwards. The acromial part of deltoid is the strongest shoulder abductor. Milch's paradox63 refers to a transformation of the muscular system around the shoulder, from a haphazard distribution in the resting position with the arm hanging, to an organized display of conical symmetry between the muscles of the humerus, scapula, and thoracic wall when the arm is completely abducted.64 While Dr. Henry Milch presented this as an argument against reduction maneuvers with the arm in the anatomic position, it is also evidence of the development of the shoulder joint when we used to climb trees millions of years ago.
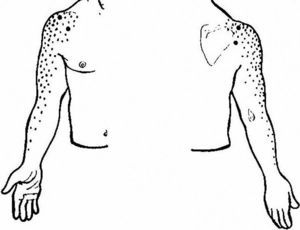
Peripheral neuropathies that cause shoulder pain, the axillary nerve neuropathy and the suprascapular nerve neuropathyPatient 7. Axillary neuropathy “A 56 year-old male has lateral shoulder pain that begun abruptly 2 days after playing tennis. Pain is continuous and burning. An area of hypoesthesia is detected in the deltoid area.”
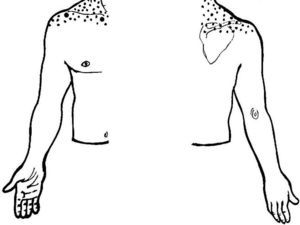
Patient 8. Suprascapular neuropathy “A 57 year-old male has posterolateral right shoulder pain. Pain is continuous and has a burning quality. Pin-prick sensation is normal.”
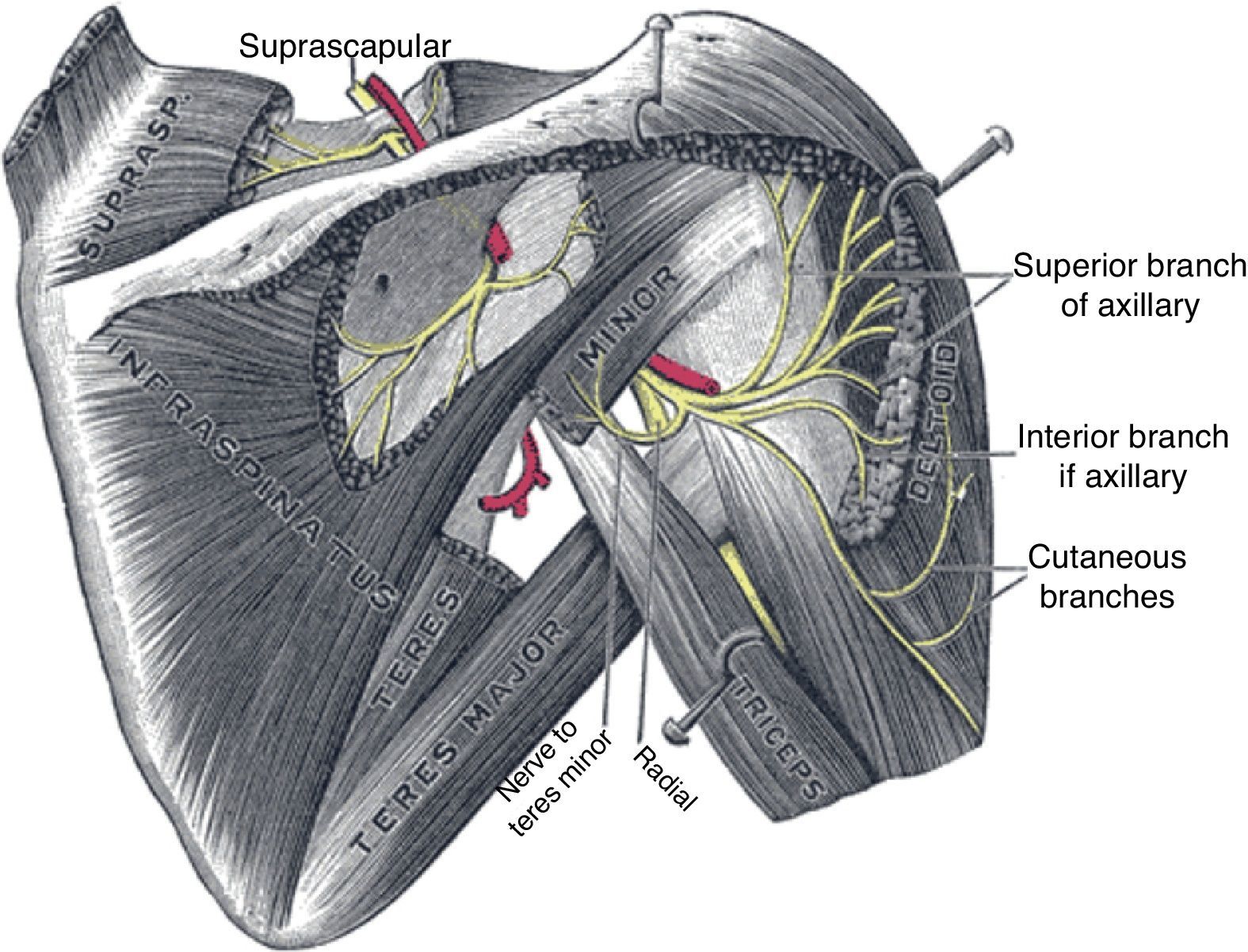
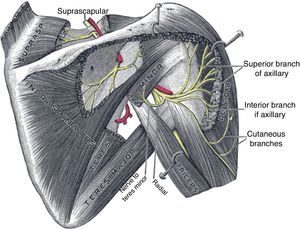
The axillary nerve derives from the posterior cord of the brachial plexus, descends anterior to the subscapularis muscle and reaches the axilla. It then winds posteriorly below the glenohumeral joint, together with the posterior circumflex humeral artery, and reaches the posterior area of the shoulder through the quadrilateral space, which is formed by teres minor superiorly, the humerus laterally, teres major inferiorly and the long head of the triceps medially (Fig. 15).65 This nerve provides motor innervation to the deltoid and teres minor muscles but also carries cutaneous sensory fibers to an oval-shaped area in the lateral shoulder.66 Most cases of axillary neuropathy are related to glenohumeral dislocations and proximal humeral fractures. The quadrilateral space is seldom the site of axillary nerve entrapment.67 The clinical features of axillary neuropathy include relentless burning pain and sensory loss over the lateral shoulder. When sought upon, loss of tone of the deltoid muscle, and selective weakness during abduction and external rotation are often detected. Late in the evolution there is a complete loss of deltoid mass and function. Fortunately, a frequent cause of this syndrome is amyotrophic neuralgia, in which after months there is a full restoration of the muscle. A concern that is sometimes raised in performing lateral subacromial bursa injections is the possibility of damaging the axillary nerve. However, the nerve encircles the humerus at a main distance of 7cm from the acromion68 and therefore far away from the usual site of injection, that is subacromial.
Suprascapular neuropathyThe suprascapular nerve originates from the upper trunk of the brachial plexus. It descends through the posterior cervical triangle along with the suprascapular vessels. It reaches the posterior face of the scapula by traversing the suprascapular notch (Fig. 15). Here, running beneath a fascia69 it provides branches to the supraspinatus muscle and curves around the lateral border of the scapular spine (spinoglenoid notch). Below the scapular spine it provides two motor branches for the infraspinatus muscle.70 The suprascapular nerve carries sensory fibers from the glenohumeral and acromioclavicular joints71 and provides motor supply to the supraspinatus and infraspinatus muscles. Injury of this peripheral nerve most often occurs due to entrapment at the suprascapular notch under the superior transverse scapular ligament and less often at the spinoglenoid notch under the inferior transverse scapular ligament or by compression by a ganglion cyst.67,69 The clinical picture of suprascapular neuropathy varies according to the site of nerve compression. Shoulder pain is prominent when impingement occurs at the suprascapular notch, along with weakness of the supraspinatus and infraspinatus muscles. When nerve injury occurs at the level of the spinoglenoid notch, a painless weakness of external rotation and atrophy of the infraspinatus will occur.67
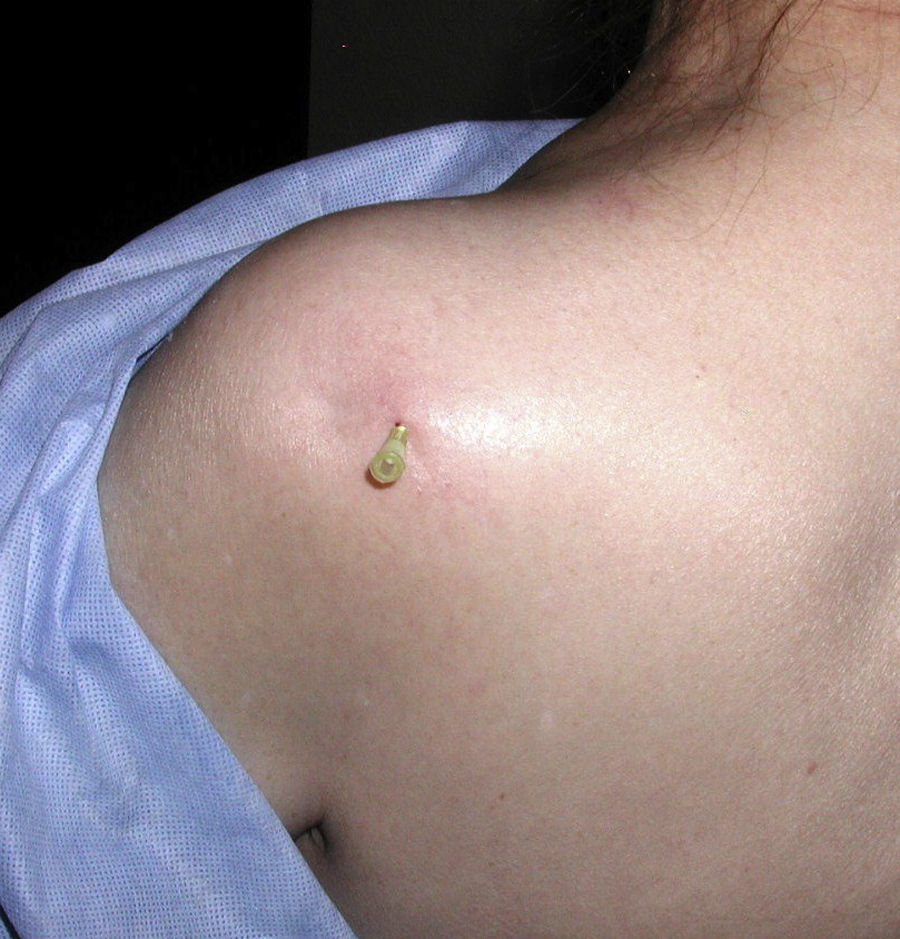
Posterior injection of the shoulder jointThe technique is quite simple. First, the coracoid process must be located. Next, the posterior angle of the acromion. A mark is made in the skin 1cm below and 1cm medial to this angle. This is the needle entry. The needle, that must be at least 35mm in length, is inserted toward the coracoid behind a front of anesthesia (Fig. 16). Bone contact indicates the humeral head. The needle is moved back 1mm and the steroid injection is placed.30
- •
Functional plane of shoulder
- •
Scapulothoracic motion
- •
Glenohumeral motion
- •
Sternum
- •
Clavicle
- •
Acromion; its posterior angle
- •
Scapula; spine and angles
- •
Deltoid muscle; origins, insertion and actions
- •
Sternoclavicular joint and its motion
- •
AC joint and its motion
- •
Coracoid process
- •
Coracohumeral ligament, can it be palpated?
- •
Greater and lesser tubercles
- •
The bicipital groove
- •
Rotators cuff
- •
Supraspinatus
- •
Infraspinatus
- •
Teres minor
- •
Subscapularis
- •
Rotators interval
- •
Axillary nerve, “safe area” for injections, surface innervation
- •
Suprascapular nerve
The authors have no conflict of interest to declare.