Cogan's syndrome is a rare autoimmune disease that usually affects young Caucasian adults and is classically defined as the combination of nonsyphilitic interstitial keratitis and audiovestibular symptoms resembling Meniere's disease, both of them developed in an interval of less than two years. Nevertheless, cases with atypical ophthalmologic and audiovestibular features, with systemic manifestations or affecting children and older patients have also been reported, expanding the clinical spectrum of Cogan's syndrome.
Herein, we present the case of a late-onset Cogan's syndrome associated with a large-vessel vasculitis.
El síndrome de Cogan es una enfermedad autoinmune rara, que afecta frecuentemente a pacientes jóvenes de raza caucásica y que se define clásicamente por la combinación de queratitis intersticial no sifilítica y síntomas audiovestibulares similares a una enfermedad de Ménière, que se desarrollan en un intervalo de menos de 2 años. Sin embargo, se han descrito casos con manifestaciones oftalmológicas o audiovestibulares atípicas, con síntomas sistémicos o que afectan a niños o pacientes ancianos, expandiendo de este modo el espectro clínico del síndrome de Cogan.
Presentamos aquí el caso de un síndrome de Cogan de inicio tardío asociado con una vasculitis de gran vaso.
Cogan's syndrome (CS) is an uncommon autoimmune systemic disease classically described as a triad of nonsyphilitic interstitial keratitis, audiovestibular symptoms resembling Meniere's disease, and an interval between ophthalmologic and auditory manifestations of less than 2 years,1 and it usually affects young Caucasian adults with no gender-specific prevalence.2,3 Nevertheless, cases with atypical and systemic manifestations, including large-vessel vasculitis, and affecting children and older patients have also been reported,4,5 expanding the clinical spectrum of this rare disease.
Herein, we present the case of a late-onset CS associated with a large-vessel vasculitis.
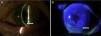
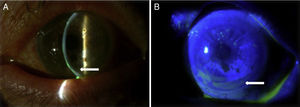
Case reportA Caucasian 82 year-old woman, with a history of gluten intolerance, osteoporotic hip fracture under treatment with risedronate and vitamin D, and bilateral dacryocystitis and cataracts, attended our outpatient clinic remitted from the department of Ophthalmology of our hospital to evaluate joint pain. She presented recurrent episodes of bilateral peripheral ulcerative keratitis (PUK) with corneal thinning for three years. A year after the onset of ocular complaints, she progressively developed a bilateral sensorineural hearing loss with impaired word discrimination and dizziness over months. Likewise, she related recurrent episodes of erythema nodosum on the legs, oral ulcers, low-grade fever and arthralgia of ankles, knees and wrists accompanying the episodes of keratitis. On physical examination, the patient had oral ulcers and arthritis of left wrist and right knee. Slit-lamp examination showed ciliary hyperemia, PUK and corneal thinning (Fig. 1), with no other relevant findings. The rest of the physical examination was unremarkable.
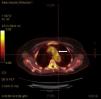
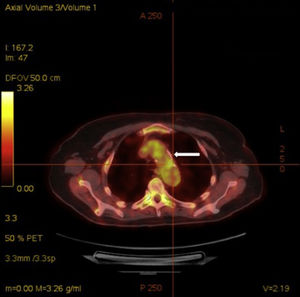
Acute phase reactants showed a mild elevation (erythrocyte sedimentation rate 26mm [normal values 0–20mm], C reactive protein 2.1mg/dL [normal values 0–0.5mg/dL]). Additional laboratory tests, including complete blood count, urinalysis, serum electrolytes, creatinine, liver transaminases, angiotensin converting enzyme, HLA-B5, antinuclear antibodies, rheumatoid factor, anti-citrullinated protein antibodies, antiphospholipid antibodies, antineutrophilic cytoplasmic antibodies and anti-heat shock protein 70 antibodies, were negative. Infectious workup for Mycobacterium tuberculosis, Treponema pallidum, Chlamydia, Borrelia burfdorferi, hepatitis B and C viruses, and human immunodeficiency virus was also negative. Plain radiographies of chest, hands, feet, and knees, brain magnetic resonance and transthoracic echocardiography showed no relevant findings. Finally, a 18F-fluoro-desoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) showed an increased metabolic activity of thoracic aorta and subclavian arteries (Fig. 2) with a maximum standardized uptake value of 2.72g/mL and 2.80g/mL, respectively. The patient was diagnosed with atypical CS based on the presence of ophthalmologic, audiovestibular and systemic symptoms, and an interval between the onset of ophthalmologic and audiovestibular manifestations of less than 2 years, although it has to be acknowledged that hearing loss could be also attributed to degenerative changes as a result of aging. Due to the high risk for the development of glucocorticoid-induced toxicity in this patient, low-dose prednisone (10mg per day) and subcutaneous methotrexate (15mg per week), as a steroid-sparing therapy, were prescribed with resolution of systemic manifestations and a mild improvement of keratitis. Hearing loss remained unchanged.
DiscussionThe diagnosis of CS is based on the characteristic ophthalmologic and audiovestibular manifestations, after exclusion of infectious and other immune-mediated diseases (e.g., tuberculosis, syphilis, sarcoidosis, Takayasu disease or granulomatosis with polyangiitis),6 and criteria proposed by Haynes et al.7 can be helpful. This fact, along with the uncommonness of the entity, the absence of appropriate diagnostic tools and non-specific clinical manifestations at disease onset, often delay the diagnosis of CS.6 Therefore, a high clinical suspicion is essential to consider CS in the differential diagnosis of patients with symptoms of ocular and/or audiovestibular inflammation. As shown in the case described above, temporal aggregation of symptoms is a diagnostic clue. Additionally, the clinical spectrum of CS has expanded along the years with the description of new systemic or atypical manifestations, or cases of children or older patients,4,5 adding complexity to the diagnostic process. The previously described case, affecting an old woman with florid systemic expression, would be an illustrative example of the expanded clinical spectrum of CS. Within the associated systemic manifestations, it is worth remark the presence of vasculitis, which is considered by some authors as the underlying pathological mechanism responsible for this syndrome.8 The role of 18FDG-PET/CT to evaluate underlying vasculitis in CS patients is still under investigation,9 even though it unveiled aortitis in our patient, a finding that reaffirmed us in our initial clinical suspicion.
The main reason to establish an early diagnosis of CS is to initiate an early therapy in order to prevent irreversible damage of eye and ear, and systemic complications. Classically, glucocorticoids have been considered as the first-line therapy,10 and immunosuppressant agents, such as methotrexate, azathioprine, cyclophosphamide, cyclosporine or tacrolimus, are prescribed in steroid-resistant CS or due to unacceptable side effects of glucocorticoids.10 Novel biological therapies, e.g., etanercept, infliximab, rituximab and tocilizumab, have been tried in CS patients, with promising results.11–13 However, no conclusive therapeutic recommendations can be stated, since the rarity of CS precludes any attempt to perform randomized clinical trials.
ConclusionCS is a rare autoimmune disease that combines ophthalmologic and audiovestibular manifestations, frequently developed in a short period of time. A high clinical suspicion has to be taken into account to consider this entity in the differential diagnosis of patients with symptoms suggestive of ocular or audiovestibular inflammation, even though the clinical picture differs from the classically described.
An early diagnosis is essential to initiate an early treatment in order to prevent damage of eye and ear, and systemic complications.
Ethical disclosuresProtection of human and animal subjectsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of dataThe authors state that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the correspondence author.
Conflict of interestNone declared.